62 Thermal emission and blackbody radiation
Any material with a temperature above absolute zero will have oscillating charged particles and thus emit electromagnetic radiation, a process called thermal emission. For objects at room temperature, this radiation is in the infrared wavelength band. For objects as hot as stars, this radiation is in the visible and ultraviolet bands.
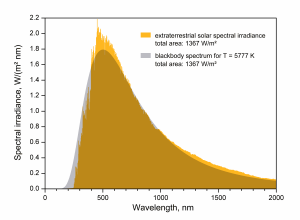
Theory exists for the intensity of the emitted radiation as a function of wavelength – the Blackbody Radiation Laws. The phrase “blackbody” may seem strange – it is meant to describe an object that absorbs all incoming radiation regardless of wavelength, i.e., there is no surface reflection. Planck’s Law specifies the theoretical function of wavelength for the thermal emission intensity from such a blackbody.
The Planck function has a prominent peak in the spectrum of emission intensity, with a temperature dependence that is given by Wien’s Law,
![]()
As the units suggest, the temperature must be specified in degrees Kelvin (K) = degrees Celsius + 273.16.
The total energy flux is also temperature dependent. Integrated over all wavelengths the total emission intensity is given by the Stefan-Boltzmann Law,
![]()
Below is an interactive calculator for the distribution of emitted energy as a function of wavelength – the Planck function – by PHET Interactive Simulations at the University of Colorado Boulder.
Media Attributions
- EffectiveTemperature © Wikimedia: Sch is licensed under a CC BY-SA (Attribution ShareAlike) license

