11 Cell Cycle
Pamela Langer
Cell Cycle Session Learning Objectives
SLO 1: Summarize the cell cycle and the events that occur in Go, G1, S, M, G2, and M phases.
SLO 1: Summarize the cell cycle and the events that occur in Go, G1, S, M, G2, and M phases.
Cell Cycle Overview
Regulation of the cell cycle is crucial for normal development and proper maintenance of tissues. Loss of this regulation is one of the fundamental defects leading to the onset of cancer. Either overstimulation of cell cycle progression or avoidance of cell death via apoptosis can lead to uninhibited cell growth and propagation. Cell signaling pathways regulate molecular choices that tip the fates of cells. For an interactive review, visit Howard Hughes Medical Institute (HHMI) BioInteractive module “Cell Cycle Regulators and Cancer” at: https://www.hhmi.org/biointeractive/eukaryotic-cell-cycle-and-cancer.

Fig. 1. Cell Cycle (Lehninger ed. 3, Fig. 13-30)
- Length of cell cycle phases varies among different cells and conditions
- Interphase = G1 + S + G2 phases
- Cell may enter into a G0 phase if it is terminally differentiated or in the absence of growth signals
- M phase is divided into prophase, metaphase, anaphase, telophase, cytokinesis (not shown in figure)
- Four main regulatory checkpoints are indicated as bars crossing the circle
SLO 2: Describe multiple regulators of the cell cycle, including cyclin, cyclin dependent kinase (CDK), and CDK inhibitors.
Molecular processes in regulation of the cell cycle
A network of regulatory proteins mediates an ordered series of switches that promote or inhibit cell cycle progression. It is an evolutionarily conserved system that differs in the details among organisms. Regulation relies heavily on protein phosphorylation and dephosphorylation as well as timely synthesis of proteins and degradation via the ubiquitin/proteosome system (UPS). Cell proliferation is stimulated by various signal transduction pathways that promote the progression of the cell cycle or inhibit processes that would lead to apoptosis. There are many layers of details understood with respect to the cell cycle, however we are focusing here on the larger picture of regulatory events and defects related to promoting cancer.
Cell cycle checkpoints
The most critical checkpoint in the cell cycle is the G1, G1/S or “Restriction point.” Progression through the G1/S checkpoint requires stimulation, otherwise the cell will remain in G0 (G zero), a state that does not progress to the S phase. In order to pass the restriction or R point, the cellular environment must be favorable for DNA synthesis to begin. That is, growth factors or other external or internal signals should be present to stimulate progression of the cycle, and the cell should be the appropriate size and contain proteins and other components required for DNA synthesis. If cellular DNA is damaged, the cell cycle will pause until it is repaired or undergo apoptosis if the damage is severe. Once the G1/S checkpoint is passed, the cell is committed to synthesizing DNA and preparing for cell division. However, the cell is not committed to divide at this point because later checkpoints can also stop cell cycle progression.
At the S checkpoint, DNA damage or DNA replication errors will also cause the cycle to pause here until the DNA is repaired. The protein kinase ataxia telangiectasia mutated (ATM) protein is involved in halting the cell cycle in S phase, and defects in this protein are associated with increased risk of certain cancers. The process may also involve the breast cancer antigen 1 (BRCA1) protein, a tumor suppressor protein that is defective in some breast cancers.
At the G2 checkpoint, the DNA must be finished replicating, and if there is DNA damage, the cycle will once again pause until the damage is repaired or undergo apoptosis if the damage is too great.
The M checkpoint is at the metaphase to anaphase transition within the M (Mitosis) phase of the cell cycle. It is also called the “Spindle checkpoint” because sister chromatids must be attached to microtubule spindle fibers from opposite poles of the cell in preparation for chromatid separation. Anaphase-promoting complex/cyclosome (APC/C) is a ubiquitin protein ligase that promotes the degradation of proteins holding chromatids together at the centromere in order to allow chromatid separation during cell division. To avoid confusion, it is important to note that the anaphase-promoting complex (APC/C) is a cell cycle regulatory complex, and a similar acronym is attributed to a different protein, namely the adenomatous polyposis coli (APC) protein that is encoded by a tumor suppressor gene that is often found mutated in colorectal cancer.
Cyclin-CDK regulation
A family of proteins called cyclins, and a family of protein kinases called cyclin-dependent protein kinases (CDKs), regulate progression through the cell cycle. Each CDK must be associated with a cyclin in order to be active. CDK activity is further regulated by phosphorylation. Signal transduction pathways, stimulated by external growth factors or other stimuli, or intracellular events, will lead to expression of different cyclin genes. Periodic synthesis of different cyclins regulates activity of their CDK partner, and degradation of cyclins via the ubiquitin proteasome system is an essential part of cell cycle progression. The activities of cyclin/CDK complexes are also regulated by specific CDK inhibitors (CKIs).
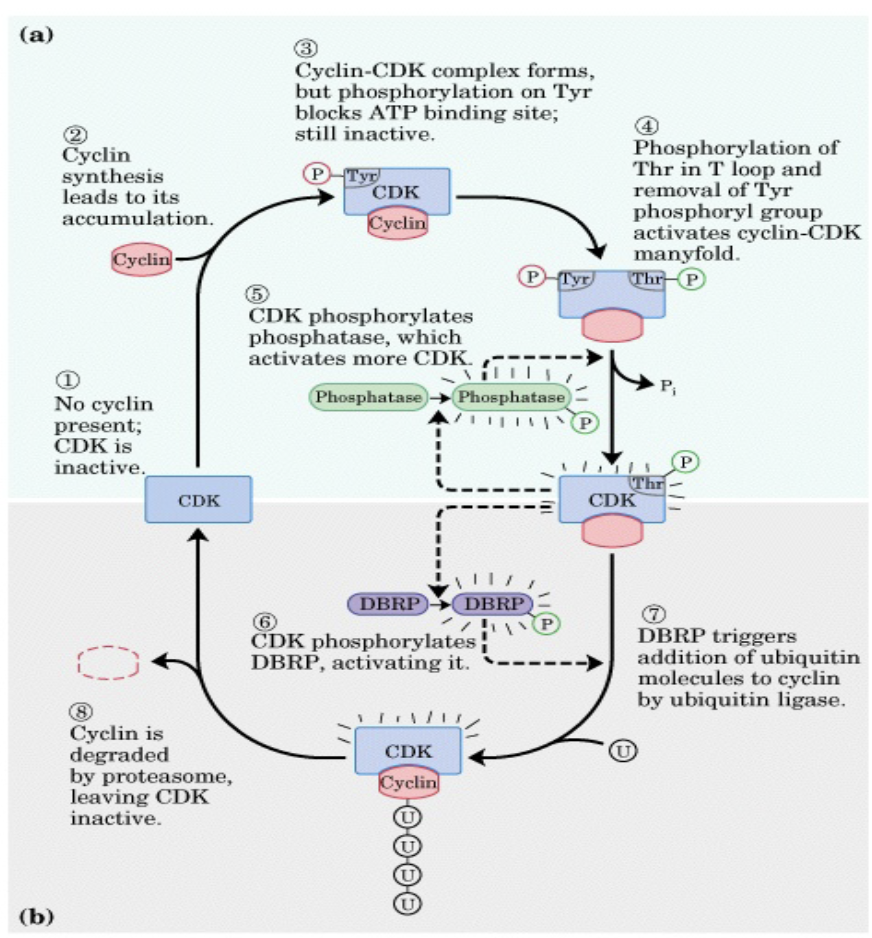
Fig. 2. Cyclin-CDK regulation summary
Figure is provided to illustrate cyclic nature of the changes in cyclin/CDK activity and is not meant to be memorized.
Signaling pathways and the cell cycle
Many different signaling pathways affect cell cycle regulation. For example, activation of a receptor tyrosine kinase (RTK) can stimulate a PI3 kinase or Ras/MAPK “pro-survival” pathway. Apoptosis of a cell is inhibited when it is receiving pro-survival growth signals. A defect in the normal apoptotic response could result in unregulated cell growth and division. A signaling pathway map in frequent use is provided here to illustrate this concept and is not meant to be memorized! However, you should note that stimulation of RTK receptors leads to multiple pathways and that this map includes pro-survival as well as pro-apototic pathways (Fig. 2).
Defects in signaling pathways can lead to unregulated cell growth and cancer. For example, defects in Ras genes can cause overstimulation of a pro-survival signal and are one of the most common oncogenes in cancers. Alternatively, a defect in a GTPase activating protein (GAP), promoting hydrolysis of the GTP that activates the Ras protein, can cause constitutive activation of the Ras/MAPK pathway if the GTP stays bound to Ras and is not hydrolyzed to GDP. One such GAP protein is the NF1 (neurofibromin 1) protein which negatively regulates the Ras/MAPK pathway normally but is most commonly associated with development of neurofibromatosis type 1 (NF1 or von Recklinghausen syndrome) when both alleles for the NF1 gene are defective in a cell. Mutations in the NF1 gene are also associated with other disorders, including some breast cancers. (Mutations in NF1, a tumor suppressor gene, are recessive, but the inheritance pattern is considered to be autosomal dominant because of the high likelihood of a second acquired NF1 mutation in cells that start out having a single NF1 mutation.)
SLO 3: Describe the roles of the retinoblastoma protein (Rb) and the transcription factor p53 in cell cycle regulation and the cancers associated with defects in these genes.
Retinoblastoma (Rb) and p53 proteins
Rb (pRb protein) and p53 (P53, TP53 proteins) play critical roles in cell cycle regulation. Rb is a type of brake on cell cycle progression. When Rb is phosphorylated, the brake is released and the cell proceeds to synthesize components required for DNA synthesis. P53 plays a dual role in cell cycle regulation in that it stimulates a process that will cause the cell cycle to pause until DNA damage is repaired or stimulates an apoptotic response if the damage to DNA is extensive. P53 and Rb are both tumor suppressor proteins, and some regulatory mechanisms mediated by P53 affect Rb activity. In brief, the increase in p53 activity after DNA damage causes a decrease in cyclin/CDK activity which results in maintenance of the Rb brake on the cell cycle. While the cell cycle is paused, the DNA damage may be repaired, thus reducing the chance of propagating errors in DNA to the next cell generation. A summary of these pathways is shown below (Fig.3.)

Fig. 3 Cyclin, CDK, pRb, E2F, p53, p21 in Cell Cycle Regulation
Hereditary Retinoblastoma
Hereditary retinoblastoma is an inherited form of eye cancer with an incidence of about 200 -300 cases per year in the US. Most cases arise in individuals who have an inherited or de novo mutation at birth in the RB1 gene encoding the Rb protein (pRb) and then acquire a second pathogenic RB1 mutation somatically. Although RB1 is a tumor suppressor gene and is recessive, the disorder follows an autosomal dominant pattern of inheritance because the risk of developing the second RB1 defect in any cell is so high. Consequently, hereditary retinoblastoma is one of the “cancer predisposition” syndromes. A child with hereditary retinoblastoma may present with a retinoblastoma in one or both eyes and has an increased risk of developing other cancers later in life. Non-heritable retinoblastoma cases, where both pathogenic RB1 alleles have developed spontaneously during a person’s life, are more likely to present as unilateral retinoblastoma.
Li-Fraumeni syndrome
Li-Fraumeni syndrome is also a rare, cancer predisposition syndrome, that results from inheritance of a single pathogenic allele of the tumor suppressor p53 gene and acquisition of a second mutation in the other, normal p53 allele in a lifetime. Individuals with Li-Fraumeni syndrome are at high risk for developing one or more independent tumors in different tissues and may elect prophylactic surgery to reduce the risk of breast or ovarian cancer for example. Patients are also advised to avoid diagnostic X-rays and any other exposure to a potential mutagen that could increase risk of DNA damage.
While individuals with Li-Fraumeni syndrome are rare, p53 mutations are some of the most common mutations found in cancers. Because p53 plays such an important role in cell cycle regulation, it is not surprising that a defect in p53 could contribute to unregulated cell growth and division, possibly even in a heterozygous state where only one p53 allele is defective.
It should be noted that as with all defective alleles associated with disorders, the clinical presentation may vary with the specific mutation that an individual has. In the case of p53, research has been aimed at correlating specific p53 mutations with the type, severity, and prognosis of cancers that develop (genotype/phenotype correlations). Furthermore, not all p53 mutations reduce or inhibit the function of the P53 protein (“loss-of-function” mutations). Some p53 “gain-of-function” mutations result in a P53 protein with increased activity that can also disrupt normal cell cycle regulatory balance.
Feedback:
