3
The Electron Transport Chain
This session continues our study of metabolism. After the initial catabolism of glucose by glycolysis, what further processing happens? When oxygen is present, how can catabolism capture more energy in the form of ATP? How does metabolism produce more metabolic intermediates essential for synthesizing other molecules (anabolism)?
Session Learning Objectives:
SLO 2. Explain how uncoupler proteins and carbon monoxide (CO) and cyanide (CN) disrupt the ETC.
AEROBIC GLUCOSE CATABOLISM – ELECTRON TRANSPORT CHAIN
SLO 1: Outline the process by which ATP is produced as a result of electron flow through the protein complexes of the Electron Transport Chain (ETC).
The electrons stored in NADH (and FADH2) produced as a result of the TCA cycle activity are transferred through a series of protein complexes of the ETC to oxygen (Figure 1, below). The electron transfer steps proceed down an electrochemical potential energy gradient, resulting in the following net reaction:
NADH + 1/2 O2 + H+ -> NAD+ + H2O
As the electrons are being transferred through the ETC, H+ are being translocated across the mitochondrial membrane by complexes I, III, and IV, resulting in the build-up of a H+ gradient across the inner mitochondrial membrane (IM), i.e. higher concentration of H+ in the intermembrane space, than on the matrix side of the IM. The change in membrane potential across the IM and proton concentration gradient provide the energy used togenerate ATP as the H+ are passed back through the ATP synthase complex (see Figure 1).
The electron carriers in the innermitochondrial membrane are oriented such that protons (H+) are pumped out of the mitochondrial matrix as electron transport takes place. The proton electrochemical gradient is used to drive the synthesis of ATP through the ATP synthase complex in the innermitochondrial membrane. Both the electrical potential difference and the proton concentration gradient that are set up provide the energy used to generate ATP as the protons are drawn back, thermodynamically downhill, into the mitochondrial matrix through the ATP synthase complex (Figure 1). Here the electrochemical gradient is harnessed to phosphorylate ATP and store energy for the cell to use. The ATP synthase couples this capture of energy. This unusual coupling of electro-chemical energy across a membrane to phosphorylate ATP is unique to mitochondria (and in plants to chloroplasts).
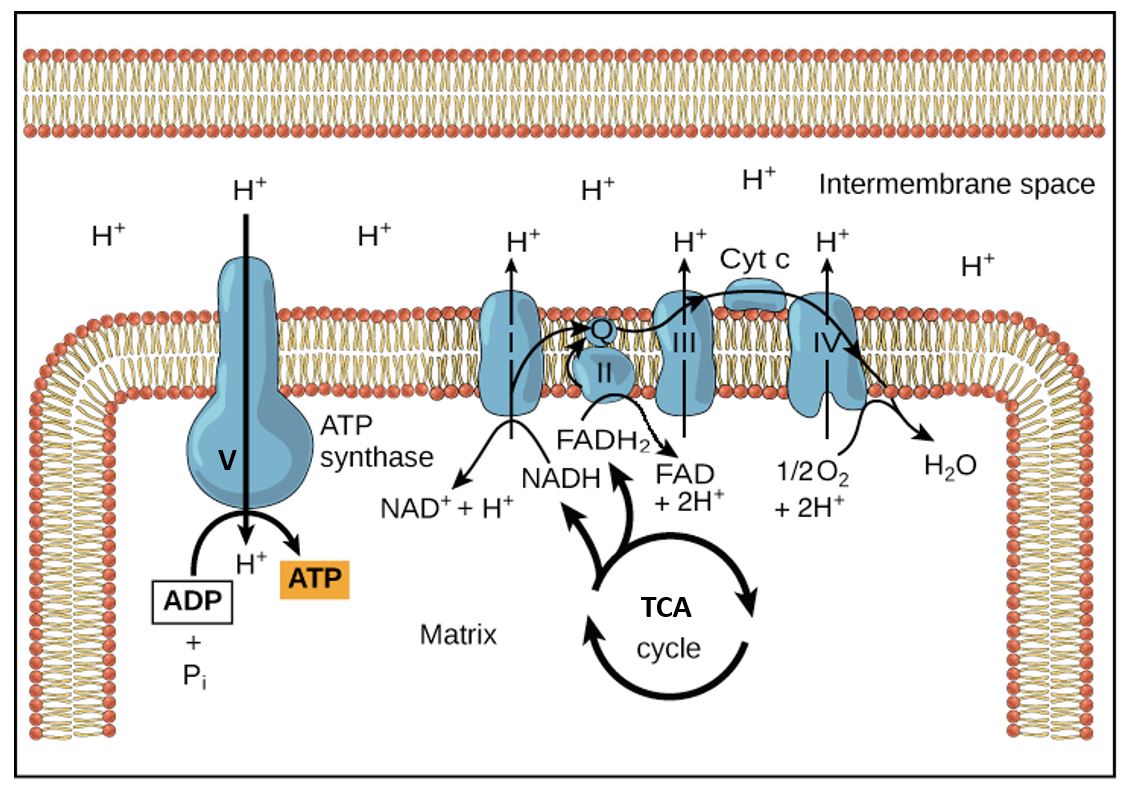
Figure 1. Disposition of ETC complexes in the inner mitochondrial membrane and flow of the reactants, electrons, and products. Complex V, the enzyme ATP synthase, couples the flow of H+ going down its electrochemical gradient to the phosphorylation of ATP. This is like a proton ATPase pump running backwards. Here the mitochondrial matrix is drawn as the top area.
The electrons are transported to oxygen along an organized series of complexes that are located within the inner mitochondrial membrane (Figures 1 and 2). The multiprotein complexes of the electron transport chain correspond to the functional names:
Complex I: NADH dehydrogenase complex
Complex II: Succinate dehydrogenase
Complex III: Cytochrome bc1 complex
Complex IV: Cytochrome c oxidase complex (contains cytochromes a and a3)
As seen above, the transport of electrons to oxygen is carried out by passage along an organized series of carriers that are located within the inner mitochondrial membrane. Coenzyme Q is lipid-soluble and transfers electrons from Complex I and II to Complex III; it is not a vitamin. This electron carrier is synthesized from the amino acid tyrosine, which is obtained from our diet. Cytochrome c, a small water-soluble protein in the intermembrane space, transfers the electrons from Complex III to Complex IV. The cytochromes are all heme proteins. Cytochrome a + a3, also called cytochrome oxidase, carries out the final transfer of electrons to oxygen.
SLO2: Explain how uncoupler proteins and carbon monoxide (CO) and cyanide (CN) disrupt the electron transport chain.
Uncoupling electron flow and O2 consumption from ATP production: Under normal circumstances, electron transport, O2 consumption, and ATP synthesis are obligately coupled through the stored proton gradient and will all slow down if the ATP/ADP ratio becomes high. However, chemical uncouplers or uncoupler proteins, disconnect the two processes by dissipating the electrochemical gradient across the mitochondrial inner membrane (Figure 2). Substrate (e.g., glucose) consumption and oxygen consumption continue, and will actually increase, because the protons are not being pushed out against a gradient and just flow right back in without doing any “work” to make ATP. Instead, the energy is released as heat rather than as ATP. In the presence of uncouplers (small molecules or proteins), protons return to the mitochondrial matrix bypassing ATP synthase. This is an important process for the regulation of energy balance and heat generation. Hibernating mammals and newborn humans use uncoupler proteins to maintain body heat.
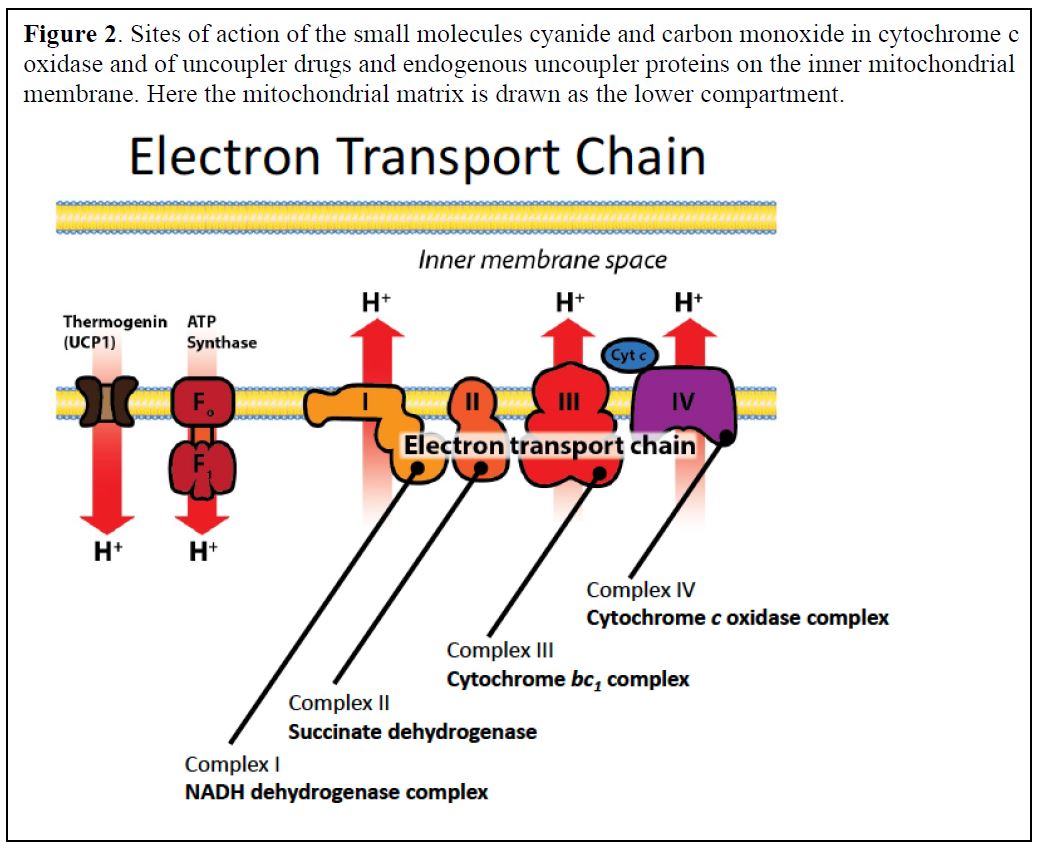
Molecules that interfere with the proper function of the ETC
Several toxic compounds bind to heme groups and inhibit cytochrome oxidase, including cyanide and carbon monoxide (Figure 3). Similarly, both poisons bind avidly to the heme of hemoglobin in competition to oxygen. Thus, they compromise respiration in several ways. Many other toxins and drugs target different complexes of the ETC. All compounds that inhibit electron transport will prevent the generation of a proton electrochemical gradient and therefore the synthesis of ATP in mitochondria. This will also result in a buildup of NADH in its reduced form, which inhibits the TCA cycle and leaves anaerobic glycolysis as the only source of ATP energy. Even though oxygen may be available, the consequences are similar to those of asphyxiation. Chemical uncouplers such as dinitrophenol increase the number of fatty acids thatmust be oxidized perATPmade, and once were used as diet pills.Natural uncoupling proteins (UCPs) were later discovered and shown to be involved with regulation of energy balance and heat generation (including in some adults).
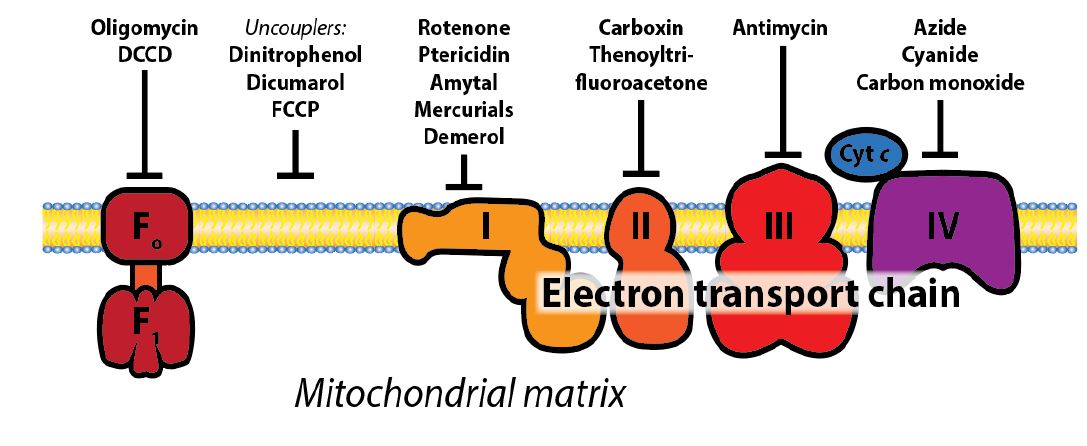
Figure 3: Schematic representation of ETC sites that acted upon by molecules that interfere with the function of the electron transport chain. Some of these are small molecules that specifically inhibit the different protein complexes of the ETC, while uncoupler proteins or molecules create holes in the membrane and destroy the H+ gradient resulting from electron flow through complex I, III, and IV of the ETC. You do not need to know the names of any of these molecules, apart from cyanide and CO.
Thought Experiment: How does the NADH generated during glycolysis enter the ETC since the mitochondrial membrane is impermeable to NAD+/NADH?. What would happen to cellular respiration if all the cytoplasmic NAD+ is trapped as NADH? Can you describe a scenario where this happens? Thought Experiment Answer.
MITOCHONDRIAL GENETICS
Mitochondria are thought to have arisen by the entry of a prokaryotic cell as an endosymbiont into an early eukaryotic cell. Mitochondria contain multiple copies of small circular DNA (mtDNA) genomes that are a remnant from this early symbiont; it encodes a few of the mitochondrial proteins involved in electron transport and oxidative phosphorylation, and some tRNA and rRNA genes. The remaining necessary genes migrated in evolution to the cell nucleus. In humans mitochondrial inheritance is through the oocyte, although there is some evidence supporting extremely rare transmission of some mitochrondria through sperm, resulting in heteroplasmy, or mixed populations of mitochondria in an individual. Mutations accumulate in mtDNA perhaps because the DNA is not packaged by histones and because mtDNA is positioned within the mitochondrion alongside the electron transport system and its byproducts, namely oxygen free radicals.
Different tissues rely on oxidative phosphorylation for energy production to varying degrees. Therefore, as mitochondrial ATP production falls with increasing severity of mitochondrial defects, there is a progressive increase in the number and severity of clinical symptoms. Mitochondrial dysfunction has been linked to numerous diseases, including diabetes mellitus, heart disease, cancer, dementia, among others. The situation is further complicated by the fact that even healthy individuals show a decline of oxidative phosphorylation with age, perhaps due to accumulating damage in mtDNA.
Practice questions on electron transport:
1.What is the biological function of the electron transport chain? Answer 1.
2.What are the advantages of respiration compared to anaerobic glycolysis? Answer 2.
3.What are the potential trade-offs of using aerobic oxidation and oxidative phosphorylation to generate ATP? Answer 3.
4.What symptoms would you expect to see in a patient with hereditary mutations in genes coding for proteins of the electron transport chain, such as hereditary defects in cytochrome c oxidase? Answer 4.
5.Would you expect a patient with coenzyme Q deficiency to be able to survive? If yes, what treatment would they need? Answer 5.
6.What unpleasant side-effects would you expect to see in a patient taking an uncoupler as a diet aid? Answer 6.
7. Assuming a cell makes a mole of ATP before and after an uncoupling molecule is added, would oxygen consumption be increased, decreased or the same? What about CO2 production? Answer 7.
8. Explain why complete oxidative metabolism of glucose is associated with only an approximate number of ATP generated. Answer 8.
ETC Quiz (1 True/False, 1 MC, 1 Drag and Drop)
The Pentose Phosphate Pathway
Definitions:
Pentose: a 5-carbon sugar such as ribose. Pentoses contrast with 6-carbon sugars like glucose and fructose, which are called hexoses.
Reactive oxygen species (ROS): Powerful oxidants that can damage biological molecules by oxidizing them. Examples are ozone (O3), hydrogen peroxide (H2O2) and superoxide (O2.-). The cell must detoxify ROS that are produced by metabolism involving oxygen.
SLO3. Explain the significance of the pentose phosphate pathway and the different roles served by the irreversible reactions of its oxidative phase and the reversible reactions of its non-oxidative phase.
Pathway Overview:
The pentose phosphate pathway (PPP), also called the pentose cycle or the hexose monophosphate pathway (or shunt), operates in parallel with glycolysis; although they both oxidize glucose, the two pathways have quite different biological roles. The product of the pentose phosphate pathway is not ATP, but reducing power in the form of NADPH which is used as an electron donor in many biosynthetic reactions and in reactions that protect cells from oxidative damage. The cycle also produces five-carbon sugars (ribose units) for biosynthesis of nucleic acids and cofactors such NAD+, FMN, and others (Figures 1 & 2).
PPP activity is minimal in muscle and the brain, where almost all of the glucose is used glycolysis for energy, ATP production. However, PPP activity accounts for a significant portion of the total glucose oxidation in tissues with active fatty acid and cholesterol synthesis, including liver. NADPH is also important for the antioxidant defenses of the body, so PPP is well developed in cells that are exposed to high oxygen levels such as the cornea of the eye, where PPP accounts for 60% of glucose oxidation, and in red blood cells.
Figure 1. OXIDATIVE PHASE OF THE PPP. The PPP has an oxidative phase (irreversible steps), which produces two NADPH, and a nonoxidative phase. PPP is regulated at the first enzyme, Glucose-6-Phosphate dehydrogenase (G6PD), activated when NADPH levels are low. NADPH is necessary for detoxification of reactive oxygen species (see below) to eliminate free radicals and for the biosynthesis of cholesterol, lipids and other products. The product of the oxidative phase, ribulose-5P, a 5-carbon sugar, is then directed to the non-oxidative phase of the PPP, which consists of a series of reversible carbon rearrangement reactions.
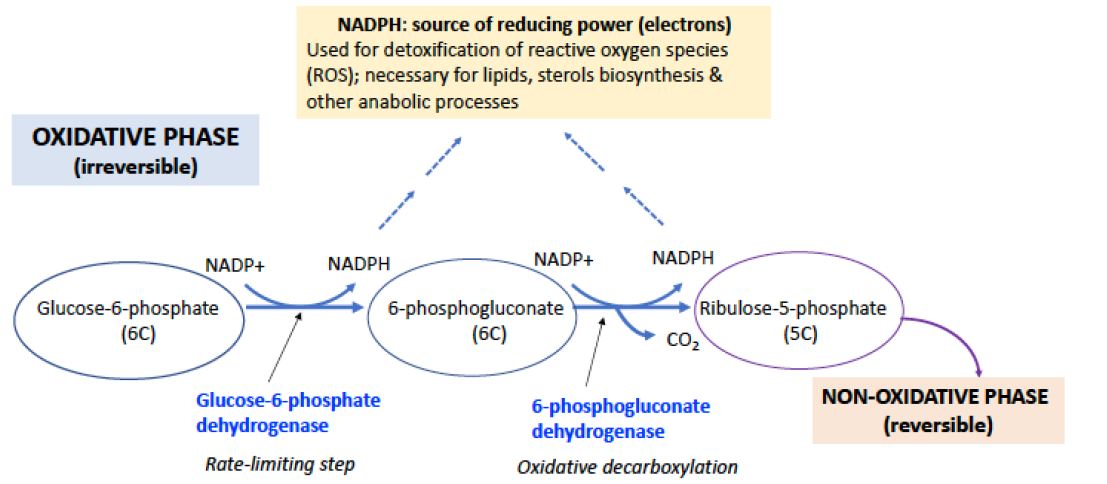
The oxidative phase of the pathway begins with glucose-6-phosphate, which is the product of the hexokinase reaction initiating glycolysis (Figure 1). Glucose-6-phosphate is oxidized to 6-phosphogluconolactone by glucose-6-phosphate dehydrogenase (G6PD) with the transfer of two electrons to NADP+ to form NADPH. The lactone is hydrolyzed to 6-phosphogluconate, which is then oxidatively decarboxylated to ribulose-5-phosphate, an isomer of ribose-5-phosphate. This step oxidizes one carbon to CO2 and generates a 2nd equivalent of NADPH, plus one equivalent of a pentose phosphate, which subsequently is available for the nonoxidative phase of the pathway (Figure 1).
The initial oxidation of glucose-6-P to 6-phosphogluconate, catalyzed by G6PD, is irreversible and is the rate-limiting step of the PPP. The ratio of NADPH to NADP+ is the main controlling factor of the reaction rate. When NADPH levels are low, G6PD is activated. The pathway is quite efficient: for example, it maintains the ratio NADPH/NADP+ at ~100 in the liver of a normal healthy individual. Under conditions where both NADPH and pentose phosphate are needed, the pathway may terminate after the oxidative phase.
Figure 2. NON-OXIDATIVE PHASE of the PPP. The non-oxidative phase reactions have double blue arrows, indicating that the reactions are reversible. Many of the sugars are denoted only by their number of carbon atoms rather than by their names here. Transketolases are key enzymes that catalyze the transfer 2 carbons, while the transaldolase catalyzes the transfer of 3 carbons. Transketolases require thiamine pyrophosphate (TPP), vitamin B1, for proper function.
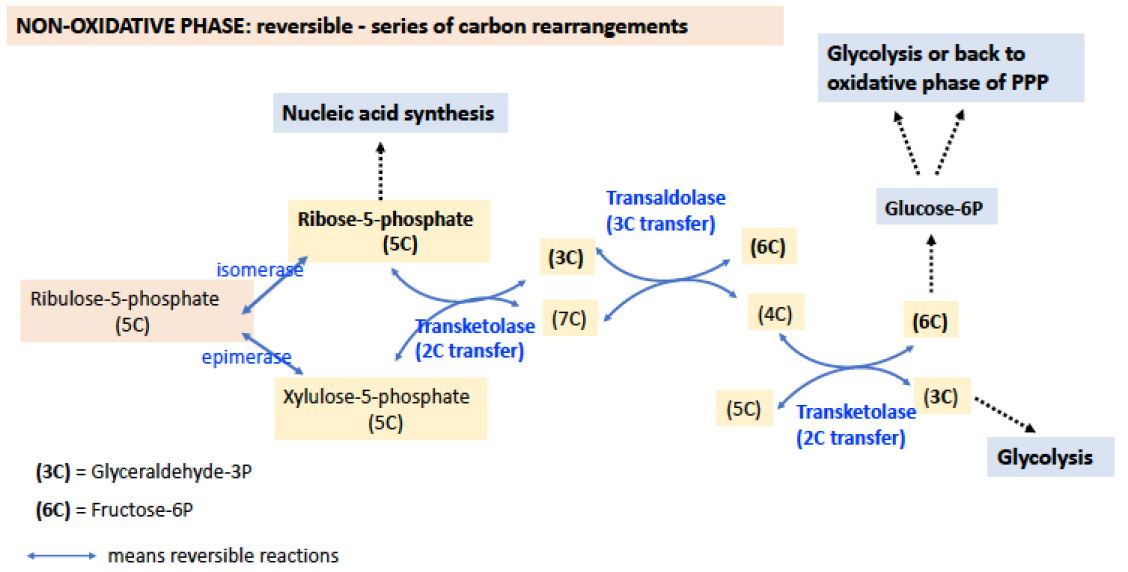
Note from this diagram how the nonoxidative phase makes several connections with glycolysis through formation of glyceraldehyde-3P, a 3C intermediate), and fructose-6-P, a 6C intermediate. It also generates other useful sugars 4C, 5C, and 7C.
NON-OXIDATIVE PHASE: Generates Ribose-5P and Series of Reversible Carbon rearrangements.
The functional role of the nonoxidative phase of the pathway is to regenerateG-6-P for the oxidative phase by shuffling carbon skeletons, interconverting 3-, 4-, 5-, 6- and 7-carbon sugars, through the action of the enzymes transaldolase (catalyzes the transfer of 3C) and transketolase (which catalyzes the transfer of 2C) (see Figure 2).
An important feature of these reactions is that they are all reversible, meaning that this phase of the pentose phosphate pathway can operate in either direction. Thus, even if there is no demand for NADPH (i.e, .NADP+ is low) and the oxidative phase of the pathway is not active, ribose phosphate can still be generated fromglucose-6-phosphate. Glycolysis and the pentose phosphate pathway share several intermediates, including G-6-P, F-6-P, and glyceraldehyde-3-phosphate. If NADPH is needed, but ribose is not (as in red blood cells), the carbon atoms are used for glycolysis.
Transketolase is one of only a few enzymes in humans that require thiamine pyrophosphate for activity. In normal individuals, a severe deficiency of thiamine (VitaminB1)will produce beriberi, a disease of diffuse symptoms associated with decreased activity of the 3 thiamine-dependent enzymes (pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and transketolase).
SLO 4. Outline how oxidative glucose metabolism and the pentose phosphate pathway are regulated to balance energy needs and NADPH production
MODES OF OPERATION OF THE PATHWAY
The pentose phosphate pathway can operate in several modes, depending on the needs of the cell. When the requirement for NADPH outweighs the need for ribose, for example in adipose tissue where there is a large demand for NADPH for fatty acid biosynthesis, the pathway operates as a cycle, generating NADPH and CO2 and returning 5/6 of the carbon to the glycolysis pathway via fructose-6-phosphate and glyceraldehyde-3-phosphate. As already noted, there are two other modes. If needs for NADPH and ribose are balanced, then only the oxidative phase can operate. If no NADPH is required, ribose-5-phosphate can be made through the nonoxidative phase of the pathway from the conversion of glyceraldehyde 3P and fructose-6P.
SLO 5. Relate consequences of glucose 6-phosphate dehydrogenase deficiency to oxidative stress, red blood cell vulnerability, and possibly malaria resistance.
SLO 6. Describe the role of NADPH in detoxifying reactive oxygen species, and explain which cell types depend the most on the pentose phosphate pathway.
Glucose-6-P-dehydrogenase (G6PD) Deficiency.
The first enzyme of the pentose phosphate pathway, glucose-6-phosphate dehydrogenase (G6PD) plays a role in avoiding oxidative stress. Inherited deficiency of G6PD is an important example of the interplay between genetics and environment. During World War II, American troops were treated prophylactically with antimalarial agents, such as paraquine or primaquine. A significant fraction of those treated suffered severe hemolytic anemia. This was later found to be an X-linked trait associated with mutations in G6PD gene. These mutations result in a substantial deficiency of the enzyme in red blood cells. Such mutations are surprisingly frequent in the human population, affecting as many as 30% of males in some Mediterranean areas and 11% of male Americans of African descent. The mutations can also result in favism, a condition in which hemolytic crisis is brought on by ingestion of fava beans, the major ingredient of falafel. The antimalarial drugs and divicine, the active ingredient of fava beans, share the common property of exposing the cells of the body to oxidative stress, from the elevated production of reactive oxygen intermediates.
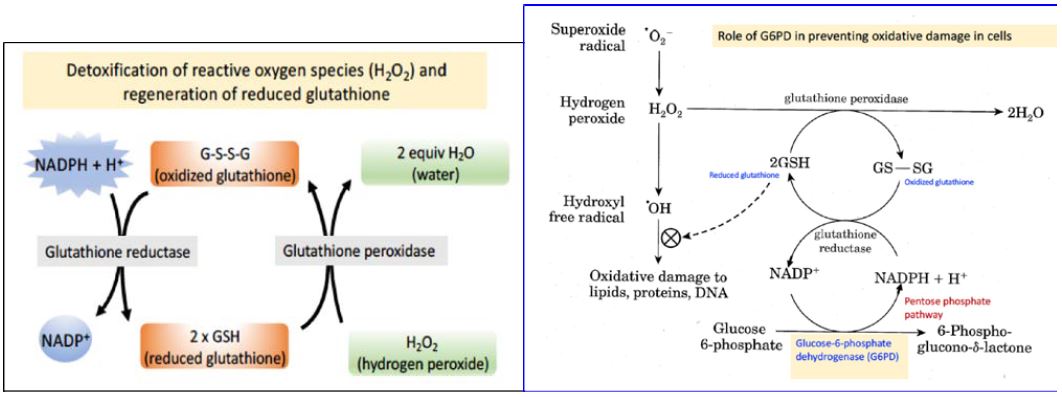
The pentose phosphate pathway is the only source of NADPH in red blood cells, and the major function of NADPH in this cell type is to maintain the tripeptide glutathione (GSH) in its reduced state, which is needed to detoxify reactive oxygen species (Figure 3).
Question: Why are red blood cells especially sensitive to mutations that create lowered levels of G6PD?
Answer: Red blood cells must live for 100-120 days without a nucleus, and therefore cannot regenerate proteins that are degraded over time. Genetic mutations can result in G6PD amino acid changes that yield a protein slightly less stable than the wild type protein. In tissues with nucleated cells, the less stable G6PD can be replenished through synthesis of newG6PDprotein.Replenishment is not an option in red blood cells, so the protein is depleted with time, generating cells that are hypersensitive to oxidative damage.
Role of reduced glutathione (GSH) in detoxification of reactive oxygen species:
GSH uses the sulfhydryl (–SH) functional group of its cysteine residue to protect cells against oxidative damage in at least two ways. First, GSH can react directly with free radicals to destroy them and thus prevent the cellular damage caused by these free radicals. Second, the enzyme glutathione peroxidase uses two electrons from glutathione to reduce hydrogen peroxide to water (Figures 3 and 4). Both of these reactions of glutathione convert GSH to the oxidized, disulfide-bonded dimer, GS-SG. Therefore, in order for glutathione to continue to be effective in combating oxidative stress, GS-SG must be reduced back to GSH. The enzyme glutathione reductase, which is quite active in red blood cells, carries out this reduction, using NADPH as a reducing agent. Therefore, defects in the ability of red blood cells to reduce NADP+ to NADPH compromise this protection system and lead to oxidative damage, ultimately causing rupture of the red blood cell membrane and hemolysis. Approximately 400 million people world-wide are affected by G6PD deficiency. Why should G6PD deficiency be so frequent in the human population? The malaria parasite, Plasmodium falciparum, reproduces in the red blood cells of infected individuals. The parasite requires glutathione for growth. Therefore, G6PD deficiency, like sickle-cell disease, confers resistance to malaria. The geographic distribution of this condition is consistent with positive selection for G6PD deficiency in areas where malaria is common.
Figure 4. Redox pathways in the red blood cell that protect against reactive oxygen species. The key position of G6PD is marked with an arrow.
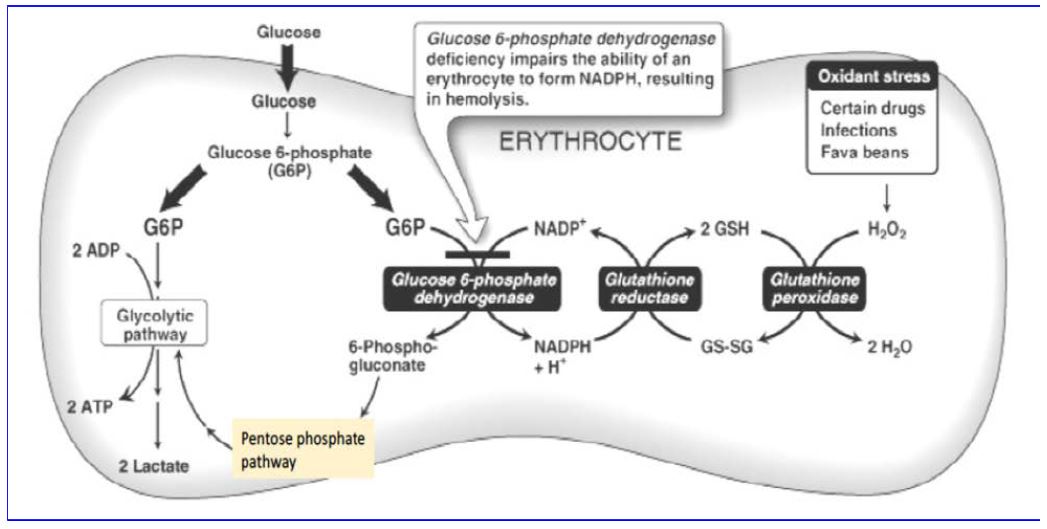
The relationship between glucose-6-phosphate metabolism and the role of glutathione in red blood cells is summarized in Figure 4. Remember that red blood cells have no mitochondria, so their only source of energy is anaerobic glycolysis.
NADPH beyond glutathione
NADPH is about much more than just reducing glutathione to protect against oxidative damage. In white blood cells NADPH is needed to create superoxide radicals to cause oxidative damage to phagocytosed microbes. All cells rely on NADPH reducing power to produce cholesterol, fatty acids and phospholipids (crucial for the plasma membrane). The liver needs NADPH to neutralize toxins (and hormones) using the cytochrome P450 pathways. Glands used NADPH to make steroid hormones.
Practice questions on the pentose phosphate pathway and reactive oxygen species:
1.What are the two main functions of the pentose phosphate pathway? Answer 1.
2.Why is the PPP so critical to the function of red blood cells? Answer 2.
3.Which tissues have high PPP activity? And what is PPP used for in these cases? Answer 3.
4.What is glutathione and what is it used for? Answer 4.
5.Why is glucose-6-phosphate dehydrogenase deficiency so prevalent in the world? Answer 5.
Feedback:
Pyruvate is reduced to lactate when cytoplasmic NADH accumulates, for example during normal anaerobic respiration. This provides NAD+ that can be used to run glycolysis. Lactate can enter the intermembrane space of the mitochondria where it is oxidized to pyruvate, and in the process reducing NAD+ to NADH. Pyruvate can be transported into the matrix, where PDH will convert it to Acetyl-CoA. The NADH (reducing power) generated when lactate is oxidized to pyruvate is transported by a malate-aspartate shuttle.
If this process is inhibited, for example by thiamine deficiency, lactate will build up. Deficits in the malate-aspartate shuttle can also disrupt oxidative phosphorylation.
Lactate can also be shuttled to the liver or other tissues where it can be used in gluconeogenesis or as an energy source.
The ETC converts reducing equivalents from NADH and FADH2 into energy, in the form of ATP.
Much more ATP is generated.
Having to handle reactive oxygen species and oxygen itself, and the resulting oxidative damage.
Patients with mitochondrial diseases, which can result from mutations in either mitochondrial chromosome encoded genes or nuclear genes that encode mitochondrial proteins, typically manifest with neuromuscular symptoms. Symptoms would include weakness, tiredness and in some cases could include epilepsy and myotonias.
Coenzyme Q is required to shuttle electrons from complex I and II to complex III. Some ATP could be produced from NADH in complex I but deficiencies reduce ATP generation and increase free radical damage. More severe deficiencies result in fatal encephalopathy and multiple systems atrophy.
Death is one of them! 18% mortality in those reporting to the ED. Heat production from uncoupling electron transport would be another- this is thought to lead to death before ATP depletion.
C02 is generated during the TCA cycle. Oxygen is the terminal electron acceptor for the ETC. Neither of these would be directly changed by uncoupling movement of hydrogen ions from the intermembrane space into the matrix from ATP production.
When NADH from glycolysis are utilized the number of ATP can vary depending on how the reducing equivalents are moved into the mitochondrial matrix.
