1
fuerstpg
GLYCOLYSIS
This session introduces the study of metabolism. Metabolism includes the processes by which the body stores and converts ingested food into energy and the many essential chemical building blocks of cells and tissues.
Metabolism also includes how substances are broken down into chemical components that are recycled or excreted. Although much of the material describes detailed enzymatic steps of biochemical conversion of key compounds, it will be equally important for you to recognize and understand a larger picture. The logic and function of the metabolic pathways can be applied to understanding how dysfunction of these pathways could contribute to common chronic diseases.
Glycolysis Learning Objectives and Brief Synopsis:
SLO1. Describe the digestion and absorption of common dietary carbohydrates.
SLO1 and 2 Quiz 1SL01 and 2 Quiz 2
Definitions:
Metabolism refers to the biochemical processes that occur within a living organism to maintain life.
Catabolism is the breakdown of complex molecules into elementary building blocks.
Anabolism is the synthesis of cellular components like RNA, DNA, proteins, lipids, and carbohydrates from elementary building blocks.
Redox reactions interconvert paired molecules (NAD+ <-> NADH and NADP+ <-> NADPH and FADH+ <-> FADH2) by reduction (gain of electrons) or oxidation (loss of electrons). Note: when NAD+ is reduced to NADH it receives two electrons. You will sometimes see this written as NADH +H, for example in figure 8. This is how NADH is able to pass two electrons when it is oxidized at the onset of the electron transport chain.
https://mediasite.hs.washington.edu/Mediasite/Play/329b89249d1e40c8b4f90719f18bf15f1d
Overview of metabolism
The breakdown (catabolism) and synthesis (anabolism) of biochemical compounds occur through separate enzymatic routes engaging a sequence of enzymes that is called a metabolic pathway.
Catabolism produces cellular energy in the form of ATP and reducing power in the form of NADH, FADH2 or NADPH. Anabolism consumes reducing power and ATP. In the body, there is continual switching between the breakdown pathways and the biosynthetic pathways to maintain homeostasis.
A key element of this switching is a reciprocal regulation of metabolic pathways that catalyze opposed processes. For example, if glucose breakdown (glycolysis) is up-regulated, the opposite pathway of de novo glucose synthesis (gluconeogenesis) will be down-regulated, a reciprocal control mechanism that prevents what are called “futile cycles.”
Energy metabolism: The main energy currency of cells is ATP, mostly generated by glucose and fat metabolism. ATP can be produced by glycolysis, and, under oxygen-rich conditions, additional ATP is produced by subsequent oxidative phosphorylation of ADP via the electron transport chain (ETC).
Products of glucose and fat metabolism enter the TCA cycle (tricarboxylic acid cycle, also called the Krebs cycle or the citric acid cycle) that generates reducing power that drives oxidative phosphorylation of ADP via the electron transport chain.
A lot more ATP can be generated through aerobic catabolism of glucose through the TCA cycle and ETC (~36 ATPs compared to ~2 ATPs per glucose molecule broken down), BUT oxygen is not always available and can be toxic as it can lead to the production of reactive oxygen species (ROS).
Strategies to make sense of what metabolic pathways mean:
First: Ask yourself what is the biological role of this pathway and what is the big picture of its activity?
Example (1): What is the biological function of glycolysis?
Answer: The role of glycolysis is to generate cellular energy in the form of ATP by breaking down glucose (a 6-carbon molecule) into smaller constituents such as pyruvate (a 3- carbon molecule)
Example (2): What is the biological function of the pentose phosphate pathway?
Answer: The role of the pentose phosphate pathway is to generate reducing power (NADPH) and to provide 5-carbon ribose sugar intermediates for nucleotide biosynthesis
Second: Remember that different tissues have different metabolic roles and different metabolic needs.
Example: The liver is an important regulator of blood glucose levels; to this end the liver can generate glucose through gluconeogenesis or by breakdown of glycogen when blood glucose levels are low. In contrast, the brain uses only glucose for ATP production and is not able to form glucose de novo. The brain is not capable of performing gluconeogenesis.
We will see more of this as we examine different metabolic pathways and processes.
Third: The key to understanding metabolism is understanding how metabolic pathways are regulated and at which levels.
Example: At the cellular level , enzyme activity can be enhanced or suppressed by the binding of enzyme inhibitors or allosteric effectors, or by covalent modifications including phosphorylation and dephosphorylation of enzymes. At the systemic level, metabolicpathwayscanbeup-regulatedordown-regulatedbyactionsofhormones.
Fourth: Remember that metabolic pathways are inter-connected.
Example: Carbons from glucose can be used to synthesize cholesterol and fatty acids. Some amino acid carbons can be converted into glucose as can the glycerol backbone of triglycerides. Other amino acid carbons and 3-carbon fatty acid remnants can enter the TCA cycle. Focus on these connections. Understand the exceptions (e.g., carbons from even numbered fatty acids CANNOT be turned into glucose).
Fifth: You are training to by physicians.
Metabolic diseases range from very common disorders like diabetes to very rare genetic disorders. In either case understanding the relationship between the affected pathway and disease will be very helpful.
METABOLISM CAN BE REPRESENTED BOTH AS BLOCK DIAGRAMS AND AS DETAILED CHARTS
Initially it is appropriate to think of glucose metabolism as composed of blocks of reactions represented as colored areas in Figure 1.
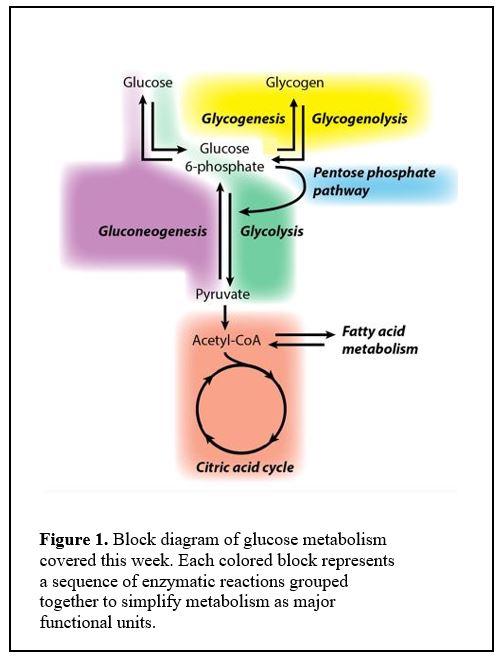
One block produces molecules that are fed into the next. For example, the glycolysis block converts glucose 6-phosphate into pyruvate, which is then converted into acetyl CoA to feed the citric acid cycle.
You should commit to memory the names of the colored blocks and how they relate to each other.
Each of the colored blocks is shorthand for a sequence of enzymatic reactions, a metabolic pathway. For many of these pathways, learning a few of the specific intermediate steps, especially those with important regulation or where an enzymatic deficiency causes a metabolic disorder, will facilitate overall understanding.
As we progress through class sessions, you can find specific reactions that we discuss in this comprehensive diagram. We will “deconstruct” the different metabolic processes and examine their inter-relationships throughout the course.
CARBOHYDRATE DIGESTION
Session Learning Objective 1. Describe the digestion and absorption of common dietary carbohydrates.
Digestive enzymes
As food passes down the alimentary tract, different enzymes are secreted for step wise digestion of carbohydrates (Figure 2 Left Panel):
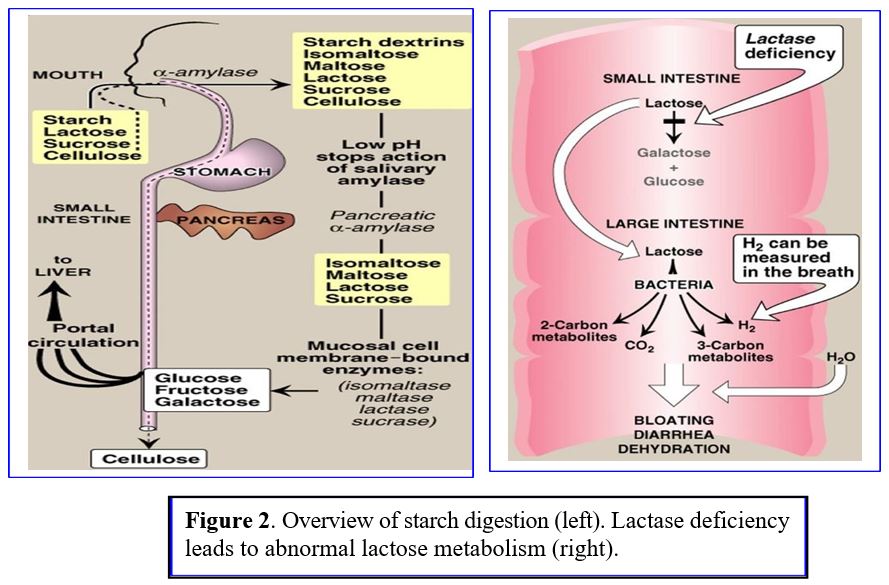
1) Salivary α-amylases break down dietary polysaccharides in the mouth; salivary amylase can partially digest starch in the stomach until the increased gastric acidity inactivates the enzyme.
2) Pancreatic α-amylases catalyzes starch/polysaccharide digestion in small intestine.
3) Additional enzymes break down complex sugars in the intestine into smaller polymers and simple sugars (monosaccharides) like glucose, fructose, galactose. Simple sugars are taken up by gut epithelial cells and rapidly shuttled into capillaries that lead to the portal venous system supplying the liver:
Sucrase: breaks sucrose into glucose and fructose, Glucose is absorbed via secondary active transport into intestinal epithelial cells; fructose enters cells by facilitated diffusion.
Lactase: breaks lactose into glucose and galactose. Lactase deficiency causes bloating and diarrhea with lactose ingestion (Figure 2 right panel). Lactose intolerance develops in > 70% of adults who lose expression of lactase with aging.
Learning objective 2. Explain how glucose is transported into and out of cells by GLUT transporters including the importance of the transporters’ relative affinities.
Glucose transport into intestinal cells. Sugars (very polar molecules) cannot cross cell membranes without the aid of transporters. Transport into intestinal cells requires secondary active transport by a Na+-glucose cotransporter (symporter) (Figure 3).
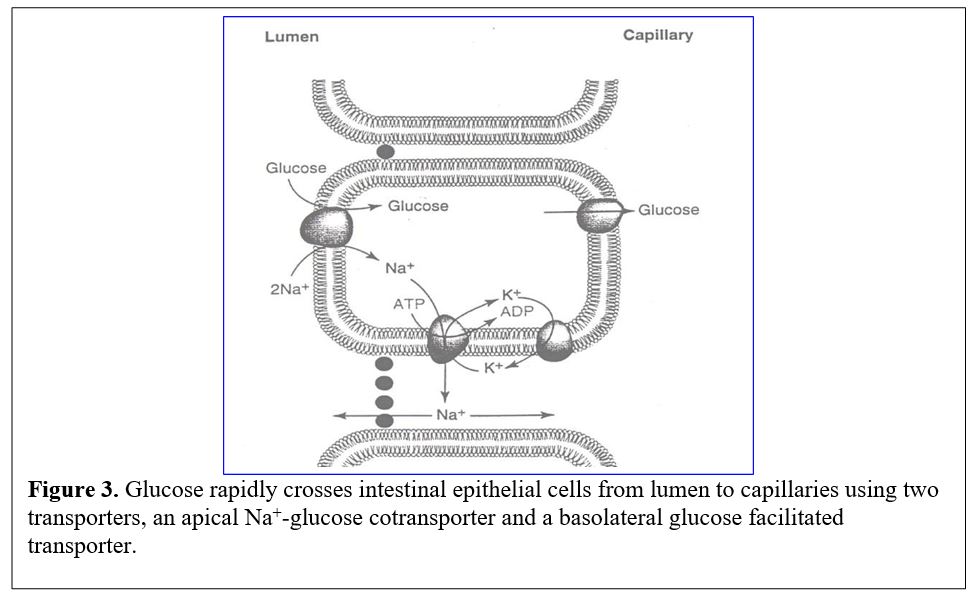 The flow of luminal glucose into the epithelial cells is driven against its concentration gradient by coupling to the energetically favorable entry of Na+ ions. Since the sodium ion gradient is maintained by the Na+/K+ ATPase pump, the energy needed for concentrating glucose can be traced secondarily to consumption of ATP. Exit of glucose from the epithelial cell into the blood stream is mediated by a glucose transporter (GLUT2) that moves glucose via facilitated diffusion and does not require the Na+ gradient.
The flow of luminal glucose into the epithelial cells is driven against its concentration gradient by coupling to the energetically favorable entry of Na+ ions. Since the sodium ion gradient is maintained by the Na+/K+ ATPase pump, the energy needed for concentrating glucose can be traced secondarily to consumption of ATP. Exit of glucose from the epithelial cell into the blood stream is mediated by a glucose transporter (GLUT2) that moves glucose via facilitated diffusion and does not require the Na+ gradient.
Principles: Enzyme Kinetics (Figure 4)
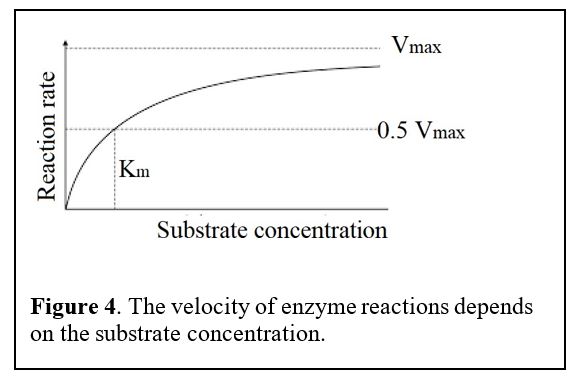 The GLUT transporters we are about to cover have different affinities for glucose.
The GLUT transporters we are about to cover have different affinities for glucose.
The velocity of transport depends on the concentration of reactants, i.e., the concentration of the substrate glucose. The velocity rises with substrate concentration but then saturates at a maximum velocity (Vmax) when every transporter becomes occupied by its substrate. We use the term Km for the concentration of substrate that gives the half maximal velocity. If the Km is small, that means that the enzyme has a high affinity for binding the substance, so it can act on its substrate even if the concentration of the substrate is low.
Notice the Km values for GLUT1-4 transporters below. Which of these has the highest affinity for glucose? Does this correlate with a low or high Km? Which transporter has the lowest affinity for glucose? Note: the Km values vary from study to study. The absolute numbers are not important but the relative affinities are (GLUT1 affinity > GLUT2 affinity etc.).
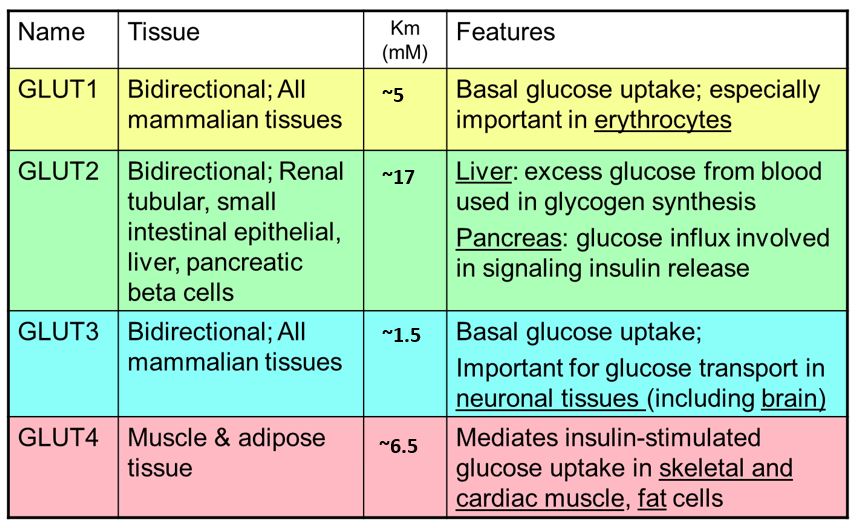
Glucose transporters: Glucose entry into most cells is mediated by multiple glucose transporters that transport glucose via facilitated diffusion. These glucose transport proteins are abbreviated GLUT (Table 1; left). Some key properties of the primary GLUT transporters:
GLUT1: these glucose transporters are found in most cell types. Because they have a low Km/high affinity for glucose, they are responsible for constitutive transport of glucose at even low blood glucose levels. GLUT1 is especially important in RBCs.
GLUT2: mediates entry of glucose into multiple cell types (see Table 1). GLUT2 has a high Km for glucose and is most important with higher blood glucose levels such as after a meal. GLUT2 has a high maximum velocity.
GLUT3: is very important in neurons/the brain. Having the highest affinity glucose transporters made in the brain ensures that glucose is reserved for the brain when glucose levels fall.
GLUT4: is expressed in muscle and adipose cells. GLUT4 regulation is especially important because it is affected by insulin, a hormone secreted by the pancreas when blood glucose is elevated. Binding of insulin to insulin receptors on muscle and adipose cells recruits intracellular GLUT4 to the plasma membrane where it promotes entry of glucose into the cells.
1. Describe the importance of Km and Vmax for different glucose transporters.
2. Why is it important that GLUT2 has a low affinity (high Km) for glucose and a high Vmax (see Figure 4 for primer on Vmax and Km)?
3. How does this affect glucose transport into the liver in the fed vs. fasted state? Why would you want neurons to have GLUT3?
4. What two types of transport are required for glucose to enter the bloodstream from digestion? Why are different transporters necessary?
Glucose entry into glycolysis
The first step of glycolysis involves phosphorylation of glucose by an enzyme called “hexokinase” and also in the liver and some other cells such as pancreatic beta cells, by “glucokinase” (Figure 5).
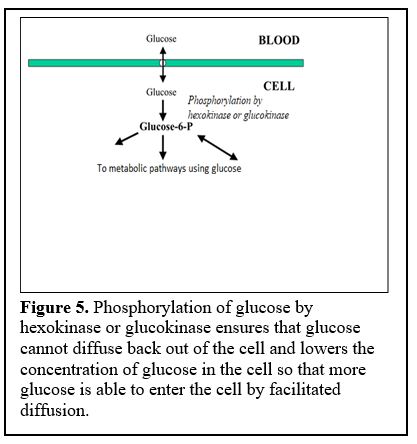
Phosphorylation ensures that glucose cannot diffuse back out of the cell.
Learning Objective 3. Explain the significance of pancreas and liver producing both hexokinase and glucokinase enzyme isoforms, and why their properties are important for these organs to regulate blood glucose levels.
There are two enzyme isoforms we will discuss that can phosphorylate glucose: hexokinase and glucokinase.
The protein isoforms (isozymes) are transcribed from different genes in different sometimes overlapping tissues. Hexokinase is broadly expressed across tissue types, including in the liver. Glucokinase is specific to tissues including the liver and pancreatic beta cells.
Hexokinase has a low Km /high affinity for glucose and is inhibited by its product, glucose-6-phosphate (G6P). Glucokinase has a higher Km for glucose, i.e. lower affinity for its substrate glucose, meaning it is active only when glucose levels inside the liver cells are high, and is not inhibited by its product, G6P (Figure 6). 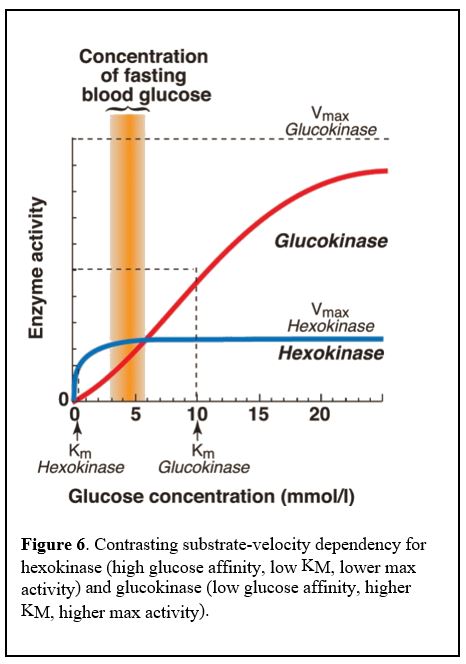
Thus, glucokinase is active only when blood glucose levels are high, normally after a meal that promotes influx of glucose into the liver cell. Glucokinase is not inhibited by the product of its reaction, glucose-6-P, so it keeps converting glucose to glucose-6-P even as glucose-6-P accumulates in the cell. This allows a heavy glucose load to be trapped inside liver cells, but only when blood glucose levels are high.
Glucokinase in pancreatic beta cells functions in a similar manner and results in the production of large amounts of ATP when blood glucose levels are high. The high ATP levels bind to an ion channel and depolarize the cell, ultimately leading to a cytoplasmic Ca2+ spike and release of insulin. Glucokinase in pancreatic cells therefore functions as the body’s glucose sensor.
1. What would happen to an individual who did not have α-amylase enzymes in their saliva, or would lack pancreatic α-amylases?
2. Why might it be beneficial for a muscle or an adipose cell to have a glucose transporter, i.e., GLUT4, that is regulated by insulin levels? When could this be important for an organism?
3. A patient with diabetes mellitus has very high blood glucose levels. The glucose enters endothelial cells through GLUT1, but the glucose concentration in the cell exceeds the level at which hexokinase reaches Vmax. What happens to (most of) the glucose?
Learning objective 4. Describe the 3 critical steps of glycolysis that are regulated and explain the significance of these three steps being regulated.
Overview of glucose metabolism
Once glucose is taken up into cells, it is converted by hexokinase (or glucokinase) to glucose-6-phosphate. Glucose-6-phosphate is a key intermediate that can go in several directions (Figure 7). It can be converted to glycogen, a polymer storage form of glucose in several tissues, including liver and muscle. It can enter into the pentose phosphate pathway (also called the hexose monophosphate pathway), producing NADPH (reducing power for biosynthetic reactions) and ribose sugars (precursors for nucleotide biosynthesis). Lastly, glucose-6-phosphate can enter into the pathway of glycolysis, which is important for energy production in all cells (most active in many cells following a meal).
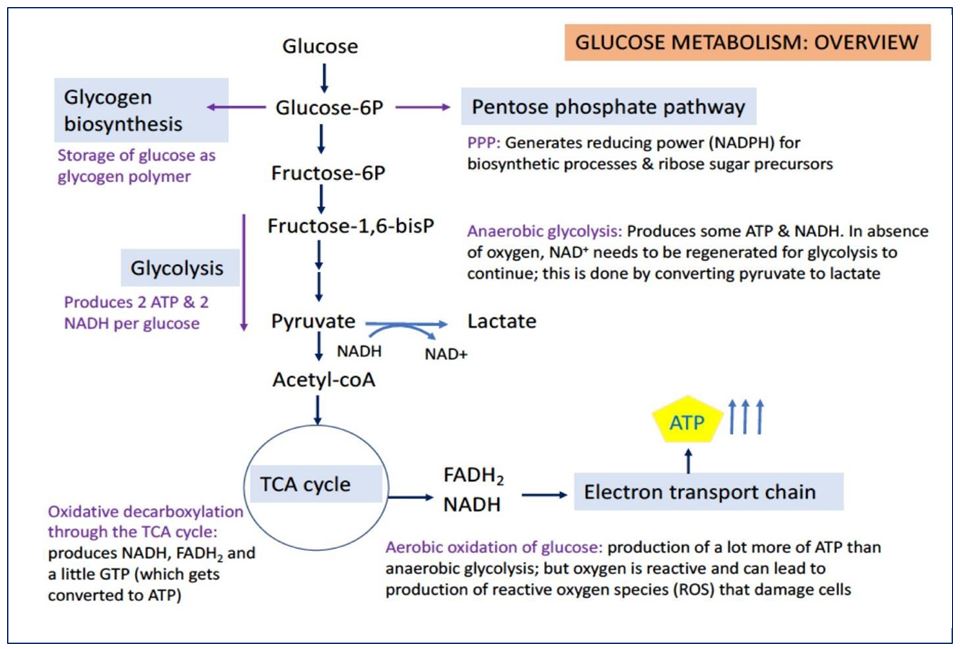
GLYCOLYSIS
Glycolysis is the breakdown of glucose (a 6-carbon sugar) into 2 equivalents of pyruvate (a 3- carbon molecule). By itself, glycolysis can provide energy to the cell. From one molecule of glucose, glycolysis yields 2 ATP, 2 NADH, and 2 pyruvates. Glycolysis is called anaerobic because it does not use oxygen.
Glycolysis consists of 10 reactions (Figure 8), including 3 key irreversible steps. These are ones to remember, so we will be going over them in detail.
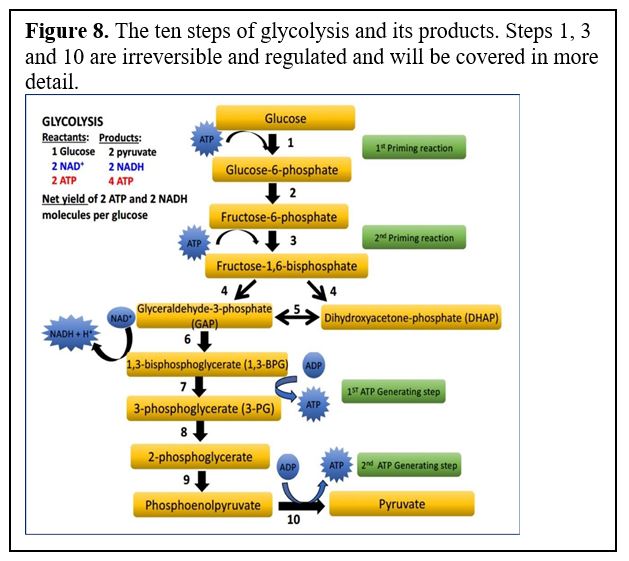 Glycolysis starts by phosphorylation of glucose followed by a second phosphorylation to yield fructose-1,6-bisphosphate (referred to as “priming reactions” in the diagram below). These two phosphorylation steps require ATP expenditure. Once fructose-1,6-bisP is formed, glucose is “committed” to break down via glycolysis, although as we will see when we cover gluconeogenesis this can be reversed by activating different enzymes when the cell needs to make glucose.
Glycolysis starts by phosphorylation of glucose followed by a second phosphorylation to yield fructose-1,6-bisphosphate (referred to as “priming reactions” in the diagram below). These two phosphorylation steps require ATP expenditure. Once fructose-1,6-bisP is formed, glucose is “committed” to break down via glycolysis, although as we will see when we cover gluconeogenesis this can be reversed by activating different enzymes when the cell needs to make glucose.
Question: “If glycolysis is used to produce energy in the form of ATP, why does the process begin by using 2 equivalents of ATP? Isn’t this a waste of energy?”
Answer:
Actually, it is not such a waste. The multiple steps in some metabolic pathways will maximize their efficiency. Here, glucose becomes a lot easier to break down into 3-carbon scaffolds if it is phosphorylated at the 1 and 6 carbon positions, therefore harnessing the energy of the cleavage reaction into subsequent ATP production steps. The priming reactions ensure that once the glucose ring is broken open, the energy can be harnessed to generate more ATP and NADH. It is thus a good strategy for the cell to start glycolysis in this fashion.
The reactions of glycolysis are catalyzed by the following enzymes. (You will not need to memorize them, but we may refer to these steps in practice questions). You should know and be able to describe the three enzymes with asterisks and how they are regulated.
*Reaction 1: Hexokinase or glucokinase (regulated irreversible step)
Reaction 2: Glucose-6-P isomerase (catalyzes a rearrangement of atoms)
*Reaction 3: Phosphofructokinase-1 (PFK-1) (regulated irreversible step)
Reaction 4: Aldolase A
Reaction5: Triose phosphate isomerase (another rearrangement of atoms)
Reaction 6: Glyceraldehyde-3-P dehydrogenase: adds a phosphate and reduces NAD+ to NADH
Reaction 7: Phosphoglycerate kinase (confusingly, the enzyme for this reaction takes its
name from the reverse reaction: 3-PG –> 1,3-BPG)
Reaction8: Phosphoglycerate mutase (catalyzes reaction similar to an isomerase enzyme)
Reaction 9: Enolase
*Reaction 10: Pyruvate kinase (again takes its name from the reverse reaction) (regulated; irreversible at physiological conditions).
Note: As we will see, when we cover gluconeogenesis, and in the glycolysis in class PPT (step 3), the irreversible steps can be reversed by different enzymes to make glucose.
The three regulated steps of glycolysis are reaction steps 1, 3, and 10. Let’s go through them one by one to highlight their significance and how they are regulated.
1st regulated step of glycolysis (reaction 1 of glycolysis): conversion of glucose to glucose-6- phosphate by an enzyme in most tissues by hexokinase and also by glucokinase in some tissues.
Advantage of converting glucose to glucose-6P using the hexokinase enzyme:
Three advantages to phosphorylating glucose by the hexokinase enzyme:
· First, it locks glucose in the cell (negatively charged glucose-6P cannot diffuse or be transported out of the membrane).
· Second, it lowers the concentration of free glucose (unphosphorylated) in the cell and therefore favors diffusion of more glucose into the cell along a concentration gradient.
· Third, this phosphorylation step requires ATP and being irreversible, permits regulation.
Thus, the hexokinase enzyme is inhibited by its product, glucose-6-phosphate. Hexokinase has a high affinity for its substrate glucose, meaning that the hexokinase enzyme is active even when glucose levels are low.
2nd regulated step of glycolysis (reaction 3 of glycolysis): conversion of fructose-6- phosphate to fructose-1,6-bisphosphate.
This reaction is catalyzed by an enzyme called phosphofructokinase-1 (PFK-1).
PFK-1 is allosterically inhibited by ATP and activated by both ADP and AMP. Thus, when ATP levels drop in the cell, and the levels of ADP and AMP concomitantly increase, the rate of this reaction will increase.
PFK-1 is also allosterically activated by an effector molecule called fructose-2,6-bisphosphate which is made by an enzyme called PFK-2.
The activity of PFK-2 is increased when the ratio of insulin to glucagon is high, while the reverse reaction is stimulated by a high ratio of glucagon to insulin. High blood glucose stimulates insulin secretion, low blood glucose stimulates glucagon secretion.
3rd regulated step of glycolysis (reaction 10 of glycolysis): Conversion of phosphoenolpyruvate (PEP) to pyruvate and generating ATP.
This reaction is catalyzed by the enzyme pyruvate kinase (PK), which catalyzes the final step of the pathway. PK is inhibited by ATP, and thus activated by a drop of ATP levels. In addition, PK is activated by the product of the PFK1 reaction, fructose 1,6- bisphosphate. Hence, when PFK1 is turned on by falling ATP levels, fructose 1,6-bisphosphate is elevated, which in turn activates PK. When ATP levels are returned to normal, the reverse of this regulatory pattern occurs and the metabolic flux through glycolysis slows down.
Take home message for regulation of glycolysis: The activity of glycolysis is regulated by the energy status of the cell. It is stimulated when ATP levels are low, and down regulated when cellular levels of ATP are high.
In addition to cellular energy status, glycolysis is regulated at the systemic level by hormones such as glucagon, epinephrine, and insulin – we will examine how these hormones regulate glucose metabolism after we have discussed aerobic glucose oxidation, gluconeogenesis, and glycogen metabolism.
Regulation Summary:
Hexokinase: Glucose-6P inhibits activity.
PF1K: F2,6BPG, AMP stimulate. ATP inhibits.
PK: F1,6BPG stimulates. ATP inhibits.
PF2K: Insulin stimulates. Glucagon inhibits.
Session Learning Objective 5. Explain how cells cope with the lack of oxygen and inability of pyruvate (end-product of glycolysis) to enter the TCA cycle and how the process of anaerobic glycolysis continues.
Glycolysis is the primary mechanism by which ATP is produced in anaerobic conditions.
In addition to generating ATP, glycolysis also generates NADH. Glycolysis cannot continue if there is not an electron acceptor (NAD+).
A reversible conversion of pyruvate to lactate by lactate dehydrogenase can be utilized:
Pyruvate + NADH <——> Lactate + NAD+
NAD+ can permit glycolysis to continue while lactate can enter circulation and be used as a fuel by other tissues that are currently aerobic. The liver can take up lactate and use it as a precursor for gluconeogenesis, thereby returning glucose to anaerobic tissues (typically muscle).
1. What would be observed in a patient who has a glucokinase deficiency?
2. What is the biological role of glycolysis?
3. What is the major bottleneck of anaerobic glycolysis?
4. What is the advantage of glucokinase having a high KM (i.e. low affinity for its substrate glucose)?
5. What would occur in a person with phosphofructokinase-1 (PFK-1) enzyme deficiency? Into which pathways would glucose be able to flow?
6. What is meant by an allosteric effector molecule?
Make sure you know which of the steps in the exercise above are regulated and irreversible!
That’s it for now! I know the class is on Monday after your first exam and have worked to make the session a good overview of the material.
Feedback

