14 Single Carbon Metabolism
SINGLE CARBON METABOLISM
WWAMI FMR
Pamela Langer, PhD, WWAMI-WY
Session Learning Objectives
SLO 2: Explain the causes and consequences of Intrinsic Factor, B12 and folate deficiencies.
SINGLE CARBON METABOLISM OVERVIEW
One-carbon fragments participate in many metabolic reactions. Single carbon donors are especially important in the biosynthesis of nucleic acid precursors, which in turn are required for cell growth and division. Because of this role in cell proliferation, single-carbon metabolism is a prominent target for chemotherapeutic drugs.
This review of single carbon, one carbon or 1C metabolism discusses the roles of vitamin B12 (cobalamin), S-adenosylmethionine (SAM), and folate derivatives as donors of 1C groups transferred in different oxidation states. For example, methyl groups are transferred from SAM and methylcobalamin; and derivatives of tetrahydrofolate (THF) are used in the synthesis of purines, deoxythymidylate (dTMP), and methionine. For this course, it is not necessary to know the specific names of THF derivative forms or which is used in each reaction. Because of the interconnected relationship of the roles of Vitamin B12, SAM and folate metabolism, a short
introduction to each will begin the discussion.
Vitamin B12 (cobalamin, B12) Figure 1
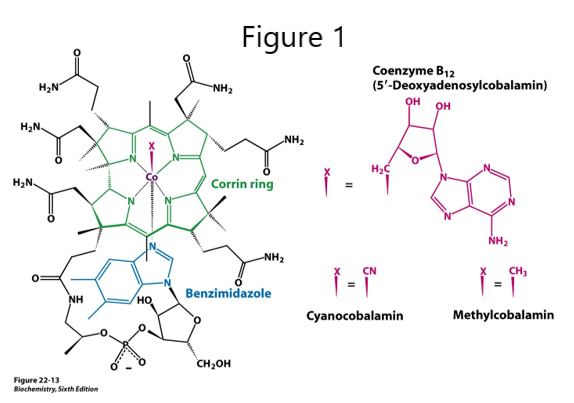
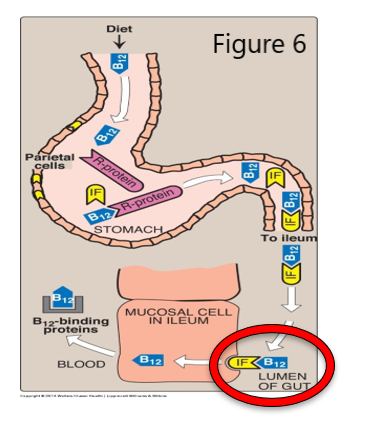
The corrin ring system of B12, related to the iron-containing porphyrin ring in heme, contains cobalt, and so, another name for B12 is cobalamin. B12 is not made by plants or animals and is synthesized only by a few species of microorganisms. B12 is derived from food sources that have harbored or eaten bacteria that make B12. Intrinsic factor (IF), a glycoprotein made by gastric parietal cells, is required for receptor mediated endocytosis of B12 during absorption in the terminal ileum of the small intestine.
Different B12 vitamers are used in the two B12-dependent reactions in mammals. Other forms of interest are hydroxocobalamin and cyanocobalamin. The low concentration of cyanocobalamin, as the B12 vitamer in many multivitamin pills, is nontoxic. Cyanide (CN) is found in insecticides, tobacco smoke, almonds, apricot kernel, apple and orange seeds, and cassava root, but symptoms appear only with high doses or long-term exposure. In CN poisoning, CN inhibits mitochondrial cytochrome C oxidase, which is part of the electron transport chain, causing a deficit of ATP along with seizure, apnea and cardiac arrest. In addition to giving a patient oxygen, hydroxocobalamin may be used in treating CN poisoning. CN displaces the hydroxyl group in hydroxocobalamin and cyanocobalamin is excreted.
Folate (Vitamin B9), Tetrahydrofolate (THF, FH4)
Animals synthesize the pteridine ring of folate but cannot conjugate it to para-aminobenzoate acid (PABA) and glutamate, so they must obtain folate from their diet or from intestinal bacteria. Folate deficiency is a common vitamin deficiency, particularly during pregnancy with the increased demands of fetal growth, and in alcoholism, with a diet often deficient in folate. However, in many (but not all) places in the world, grains and other food are now supplemented with folic acid.

Figure 2.
Dihydrofolate reductase (DHFR) catalyzes reduction of folate to dihydrofolate (DHF) and DHF to tetrahydrofolate (THF). Single carbon (1C) units, in different oxidation states, are then carried on N5, N10, or both positions in derivatives of THF.
S-adenosylmethionine (SAM)
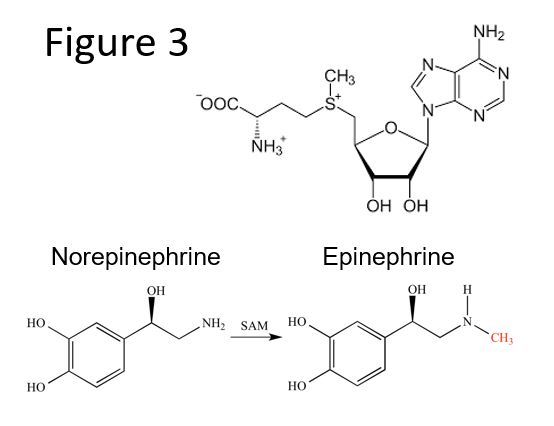
SAM (Figure 3; Top) is synthesized from methionine and ATPs. SAM contains a methyl group attached to a sulfonium ion and is a highly reactive methyl group donor. After transfer of the methyl group, SAM is regenerated in the “methyl” or SAM cycle. SAM is used by:
- Methyltransferases, in DNA methylation and synthesis of creatine, homocysteine, phosphatidylcholine, carnitine, melatonin, and catecholamine neurotransmitters (e.g. the methyl group of SAM is used in the synthesis of epinephrine from norepinephrine)
- Polyamine synthesis, utilizing a product of SAM decarboxylation, produces spermidine and spermine from putrescine.
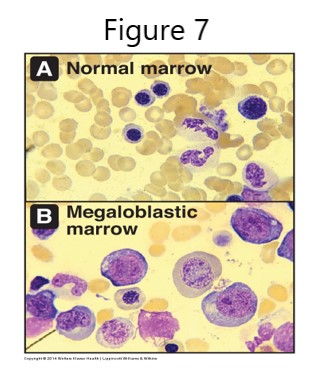
The generation of SAM and regeneration of tetrahydrofolate (THF) are connected in a folate and SAM “bicycle” with a shared reaction catalyzed by the B12 coenzyme-dependent methionine synthase. In the absence of B12, dietary methionine can still be used to make SAM, but homocysteine and N5-methyl THF will accumulate. Because the functioning of both the folate and SAM cycles is B12-dependent, B12 deficiency may result in a secondary THF deficiency. THF derivatives are required to produce precursors for DNA synthesis and repair. Consequently, a B12 deficiency can result in megaloblastic anemia. The development of megaloblastic anemia will be discussed in more detail later.
This Folate-SAM bicycle figure includes details that will be addressed throughout the discussion of B12, folate and SAM metabolism as well as in other topics in this course.
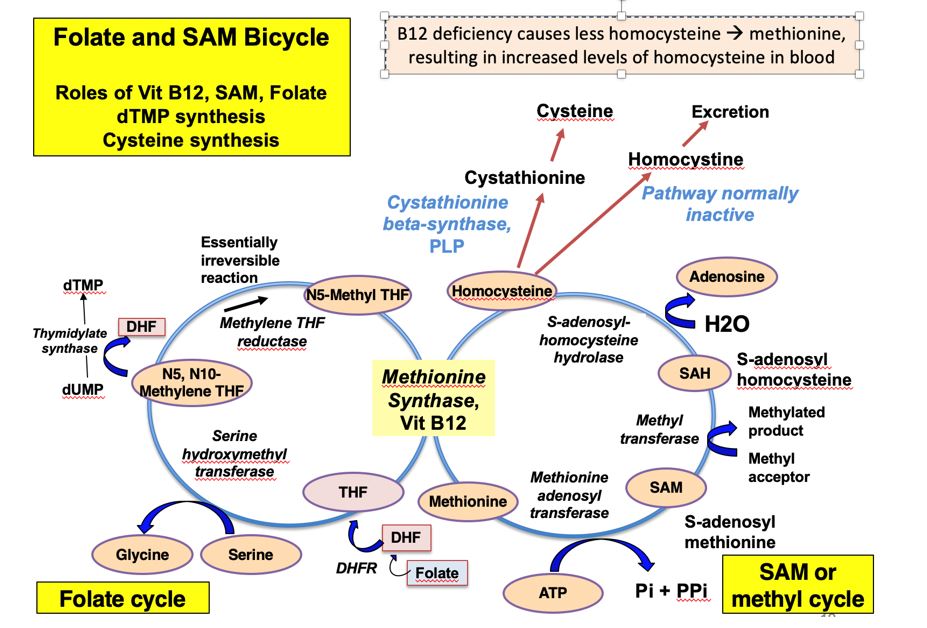
Figure 4. The Folate SAM bicycle- this one only goes forward if you pop a wheelie!
SLO 1: Identify the functions of vitamin B12, and explain the role of intrinsic factor in its absorption.
Vitamin B12 function
Vitamin B12 is used as a coenzyme in only two reactions in humans. In the B12-dependent methionine synthase reaction, a methyl group is first transferred from N5-methyl THF to cobalamin and then from methylcobalamin to homocysteine to form methionine in a so-called ‘ping pong reaction.’ The significance of this reaction is that it: (1) produces methionine, that goes on to become the important methyl donor SAM, (2) regenerates THF to sustain the folate cycle, and (3) frees the methyl group from N5-THF that would otherwise be “trapped” since the folate cycle proceeds in one direction because of the essentially irreversible reaction that generates N5-THF.
Two Vit B12-requiring reactions in mammals:
Methylation of Homocysteine to form Methionine
(Methionine synthase uses methylcobalamin)
Methylmalonyl CoA à Succinyl CoA
(Methylmalonyl-CoA mutase uses 5’-deoxyadenosylcobalamin)
Secondly, another B12 vitamer (5’-deoxyadenosylcobalamin) is required for the methylmalonyl CoA mutase reaction in the metabolism of odd chain fatty acids. Propionyl CoA arises from the breakdown of certain amino acids, cholesterol and odd numbered fatty acids. Propionyl CoA is converted to methylmalonyl CoA which in turn is converted by methylmalonyl CoA mutase to succinyl CoA, which enters the TCA cycle. A deficiency in vitamin B12 would lead to a buildup of methylmalonyl CoA, which is hydrolyzed to methylmalonic acid (MMA) and excreted in the urine of patients with vitamin B12 deficiency.
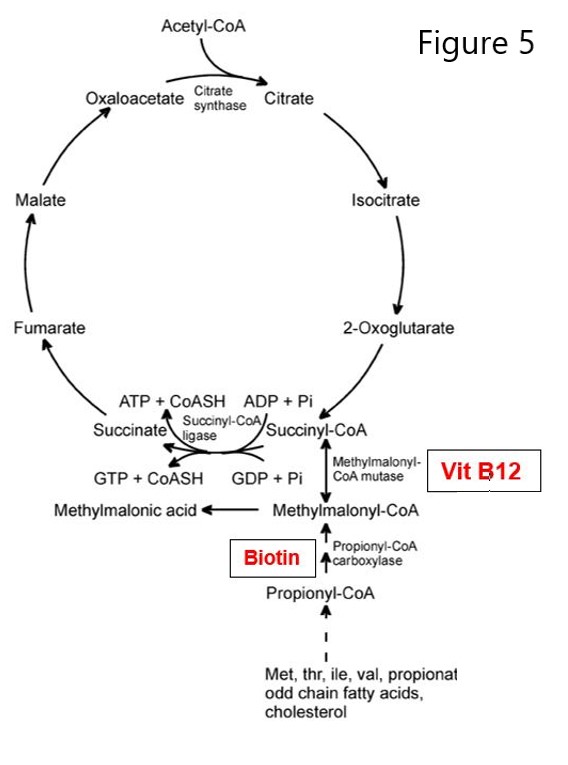
Figure 5. Methylmalonyl-CoA -> Succinyl-CoA requires B12.
People with a rare defect in the methylmalonyl CoA mutase gene present with mild to life-threating methylmalonic acidemia and very low blood pH. Because early dietary intervention may help with this genetic disorder, it is a disorder on newborn screening panels.
Role of intrinsic factor in Vit B12 absorption
- Dietary B12 is released from proteins in the presence of increased acid and proteases in the stomach.
- HCl from gastric parietal cells is necessary for production of the pepsin protease from pepsinogen.
- B12 binds an R-Protein (e.g. haptocorrin made in the salivary gland) in the mouth but mainly in the stomach, once B12 is released from food.
- Then, B12 is released from haptocorrin by pancreatic proteases in the small intestine.
- Intrinsic factor (IF), a glycoprotein secreted by gastric parietal cells, next binds B12 in the intestine.
- The IF/B12 complex goes into the enterocytes lining the ileum via receptor-mediated endocytosis.
- Once inside the enterocyte, IF is degraded, releasing B12 that will exit the cell and bind to other B12-binding proteins (e.g. transcobalamin) in blood.
- The transcobalamin/B12 complex will travel in the blood to cells where B12 functions as a coenzyme for two metabolic reactions.
SLO 2: Explain the causes and consequences of Intrinsic Factor, B12 and folate deficiencies.
SLO 3: Outline the S-adenosylmethionine and folate cycles and explain why B12 deficiency leads to megaloblastic anemia due to a secondary folate deficiency.
Decreased Vit B12 levels: some causes
A low Vit B12 level may be caused by decreased intake or availability or decreased absorption of Vit B12, for example:
- Geriatric decrease in intrinsic factor (IF): Elderly people and those with certain GI disorders may not produce sufficient intrinsic factor and therefore not absorb B12.
- Long-term reduced gastric acidity inhibits dissociation of protein bound B12
- o Proton pump inhibitor (e.g. Omeprazole) causes gastric pH ≥ 5 for 24h; pepsin is inhibited at pH 5.
- o Autoimmune attack on parietal cell H+/K+ ATPase causes reduced acid secretion. (H+/K+ ATPase pumps H+ into the gastric lumen).
- Atrophy of gastric mucosa may cause a parietal cell deficiency (e.g. with ulcers, cancer), resulting in reduced HCl and intrinsic factor (IF) deficiency
- Autoimmune attack on parietal cell intrinsic factor results in low IF, causing pernicious anemia, a type of megaloblastic anemia
- Celiac disease causes damage to intestinal lining, sometimes affecting area of B12 absorption
- Intestinal resection may involve lower ileum where B12 is absorbed
Consequences of Vit B12, folate and intrinsic factor deficiencies
Decreased release of B12 from food because of reduced acid secretion usually results in a mild B12 deficiency that may not have clinical consequences or can be treated easily with B12 injections. However, an intrinsic factor deficiency, or damage to or resection of the terminal ileum site of B12 absorption, can result in severe B12 deficiency leading to megaloblastic anemia.
Tetrahydrofolate (THF) derivatives are required for synthesis of purines and thymidylate. Synthesis is compromised when the methyl group is “trapped” in N5-methy-THF and THF regeneration is decreased. Thus, the deficiency of the THF derivative (N5, N10-methylene THF) required for dUMP -> dTMP synthesis by thymidylate synthase causes a decrease in dTTP and therefore decreased DNA synthesis and repair. The cell cycle cannot progress from G1 to S phase, resulting in continuing cell growth without division and accumulation of large nucleated RBC precursors called megaloblasts. The RBC and hemoglobin deficiencies result in reduced O2 transport and megaloblastic anemia. Other consequences of B12 deficiency may include a mild hyperhomocysteinemia associated with increased risk of cardiovascular disease, alteration of methylation reactions from SAM deficiency, and effects stemming from decreased activity of the B12-dependent methylmalonyl CoA mutase reaction.
The etiology of neurological damage associated with B12 deficiency is likely complex. Patients may present with demyelination, peripheral neuropathy, fatigue, depression and difficulty with balance. While B12 deficiency inhibits methylmalonyl CoA mutase, as well as methionine synthase at the intersection of the folate and SAM cycles, hypotheses for the mechanisms underlying neurological damage have included consequences of compromising either or both of the B12-dependent reactions. Disturbances in malonyl CoA and methylmalonyl CoA metabolism may alter fatty acid synthesis, affecting regeneration of the myelin sheath. A deficiency in SAM causes a decrease in methyltransferase reactions, potentially affecting DNA methylation and synthesis of creatine, homocysteine, catecholamines, phosphatidylcholine, carnitine, melatonin and polyamines.
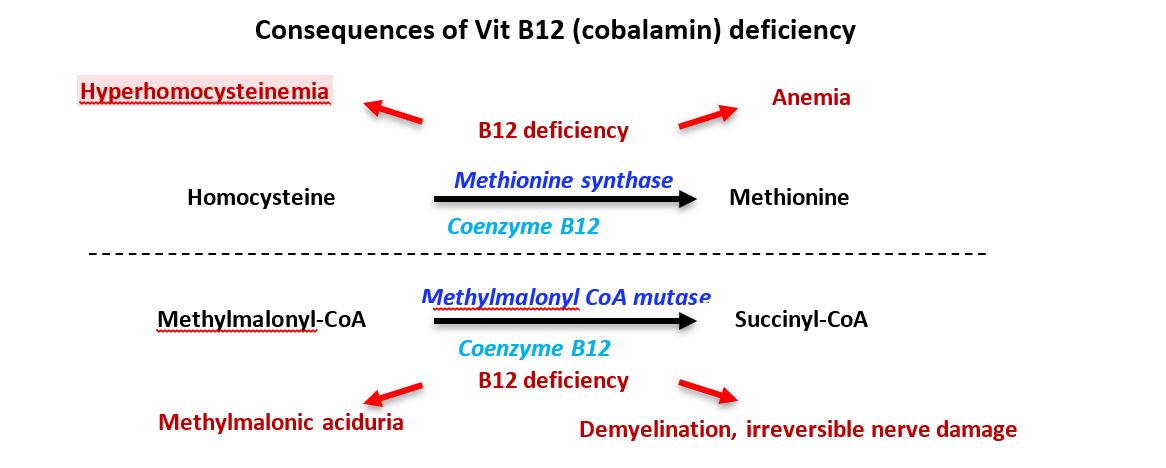
Figure 8. B12 deficiency summary
Folate deficiency in pregnancy
Increased folate is required to meet the demands of cell division, fetal growth, and altered metabolism during pregnancy. Folate deficiency can lead to birth defects such as spina bifida, a defect in closure of the neural tube. Sickle cell anemia causes an even greater need for folate. In the US, folate is added to flour and processed foods to prevent spinal tube defects caused by folate deficiency. Additional supplementation with folic acid, especially prenatally and in the first trimester, has further contributed to the decrease in neural tube defects. As a result of added folate in foods, B12 deficiency in the general population rarely causes megaloblastic anemia. However, once neurological symptoms of a B12 deficiency appear, they are irreversible. Therefore, it is important to check the B12 levels of patients at risk for other causes of a B12 deficit, such as loss of intrinsic factor.
Nitrous oxide toxicity
Nitrous oxide (N2O) oxidizes cobalt in the corrin ring, inactivating vitamin B12 but not reducing B12 levels. Chronic N2O exposure can occur from occupational, recreational or repeat (or even single) exposures in hospital procedures. Toxicity may present with unexplained neurological complications including sensorimotor peripheral neuropathy, megaloblastic changes and myelopathy such as subacute combined degeneration (SACD). For recreational users of N2O (laughing gas/hippy crack, stored in cannisters called whippets), development of symptoms depends on the number of cannisters used per day, chronicity of exposure, and if the individuals are taking Vit B12 supplements. Intermittent abuse may require several months for neurological symptoms to appear, and rarely are there hematological changes. With respect to hospital procedures, even with a B12 deficiency, N2O anesthesia will rarely result in neurological or hematological complications. With respect to clinical analytes, homocysteine and methylmalonic acid (MMA) levels may be elevated, but B12 levels may be low, normal or high. The reason for this B12 level variance is that N2O inactivates B12, but B12 level determination includes inactive and active B12.
SLO 4: Illustrate therapeutic uses of pharmaceuticals that disrupt folate metabolism and describe the consequences.
Methotrexate (MTX) is a folic acid analog that competitively inhibits dihydrofolate reductase (DHFR). DHFR normally catalyzes two reactions, involving conversion of folic acid to DHF and DHF to THF. Consequently, many metabolic processes requiring THF derivatives as coenzymes are inhibited with use of methotrexate:
- Synthesis of purine ring on ribose
- Pyrimidine biosynthesis (dUMP -> dTMP)
- Synthesis of methionine from homocysteine, via methionine synthase, and subsequently the production of S-adenosylmethionine (SAM)
Methotrexate is used as an anti-cancer drug to inhibit quickly dividing cells and also commonly used as a disease-modifying antirheumatic drug (DMARD) for rheumatoid arthritis or psoriasis. As DMARDs suppress the immune system and reduce inflammation on a time scale of weeks to months, nonsteroidal anti-inflammatory drugs (NSAIDS) are often used for more immediate pain relief. Methotrexate is sometimes used in combination with other DMARDs as the treatment strategy for rheumatoid arthritis is tailored to be the most effective for the patient. Methotrexate is on the World Health Organization (WHO) Model List of Essential Medicines.
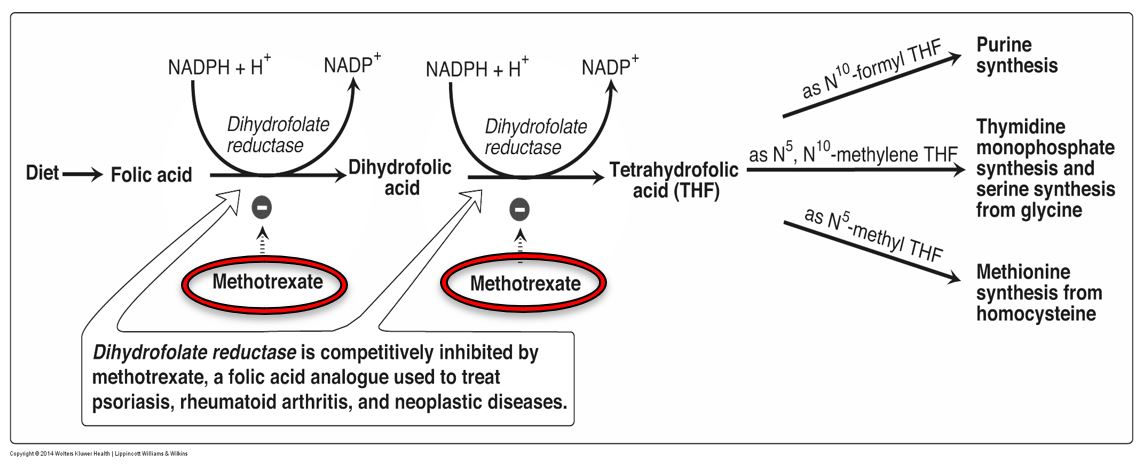
Figure 9. Methotrexate activity.
Aminopterin, another anti-cancer DHFR-inhibitor drug, was originally used to treat pediatric leukemia, but eventually its use was replaced by methotrexate, a safer, inexpensive drug. Some other DHFR inhibitors are anti-pathogen drugs, based on the principle that the drug inhibits the pathogen’s DHFR to a greater extent than human DHFR. One example of this is Proguanil that acts to specifically inhibit malarial DHFR and thus inhibit parasite replication within the red blood cell (the intra-erythrocytic stage).
Figure 10. Drug Summary 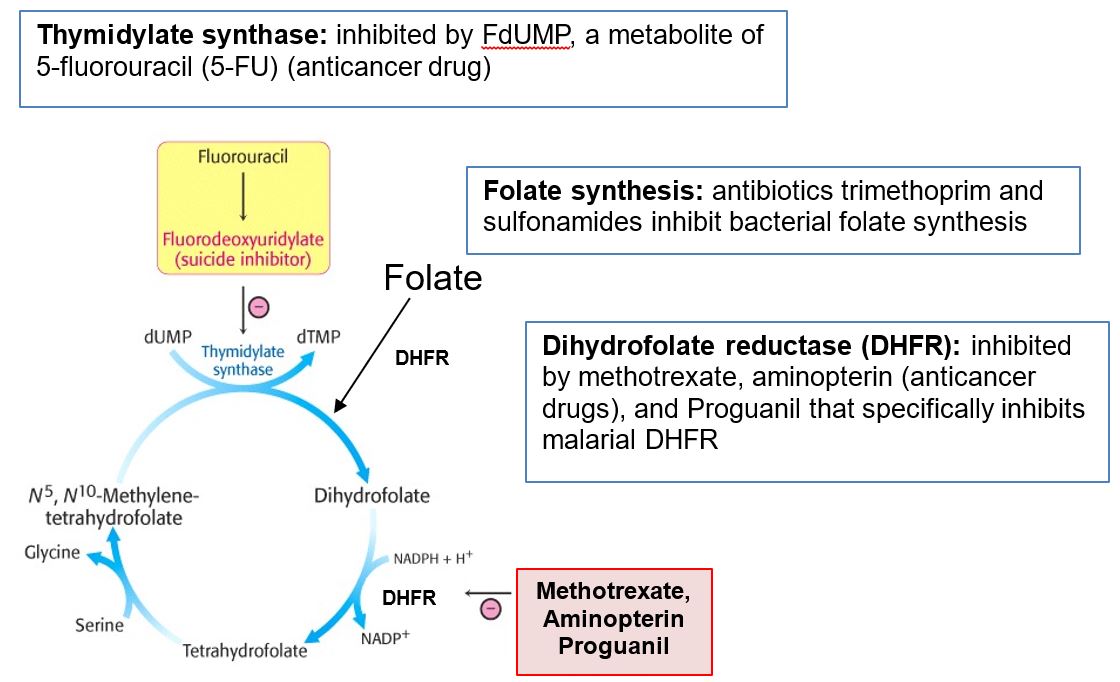
5-fluorouracil (5-FU) is used in the treatment of several cancers. This pyrimidine analog is converted to fluoro-dUMP (FdUMP, fluorodeoxyuridylate) which is an irreversible inhibitor of thymidylate synthase. The resulting deficit in dTMP leads to a deficiency in DNA synthesis and repair, as explained previously in the discussion of Vitamin B12 and folate deficiencies. The consequence of this drug therapy is inhibition of quickly dividing cells, with the target being cancer cells. However, because individuals detoxify 5-FU with different efficiencies, depending on the catabolic enzymatic variant they express, 5-FU treatment can lead to an adverse drug reaction that in some cases can be lethal. This consideration is discussed further in the sessions on purine and pyrimidine metabolism and pharmacogenomics.
Feedback:
