5 Metabolism of Fructose, Sorbitol, Galactose and Ethanol
Session Learning Objectives
FRUCTOSE METABOLISM:
SLO1: Outline the biochemical pathways involved in metabolism of fructose, its intersection with glycolysis and consequences of dietary imbalance in fructose consumption and inborn errors of metabolism related to fructose metabolism.
As a component of common table sugar (sucrose), fructose is a major source of calories in the human diet. Free fructose is also found in honey and in many fruits and soft drinks. Unlike for glucose, the uptake of fructose into cells is not enhanced by insulin, and elevated blood fructose does not stimulate insulin production by the pancreas. (A recent review on studies in the liver and kidneys reported that: 1) Fructose is converted to glucose to variable extents, depending on exercise condition, gender, and health status (range: 30-50%). 2) A portion of fructose is incorporated into glycogen after conversion to glucose, but the extent is not known. 3) Fructose affects hepatic glucose production and whole-body glucose disposal. Therefore, it is difficult to establish unambiguously how much fructose is processed into lactate or fatty acids. It may vary from one individual to the next).
The pathways of fructose metabolism are summarized in Figure 1. Fructose is phosphorylated by a specific kinase, fructokinase, to yield fructose-1-phosphate (F-1-P). In turn, F-1-P is cleaved by a special aldolase, (aldolase B), that is present only in liver and kidney tissues; it acts efficiently on both F-1-P and fructose-1,6-bisphosphate to yield dihydroxyacetone phosphate (DHAP) and glyceraldehyde. The two molecules of dihydroxyacetone phosphate produced from fructose are then either converted to
glucose (through gluconeogenesis) or further metabolized to pyruvate by way of glycolysis.
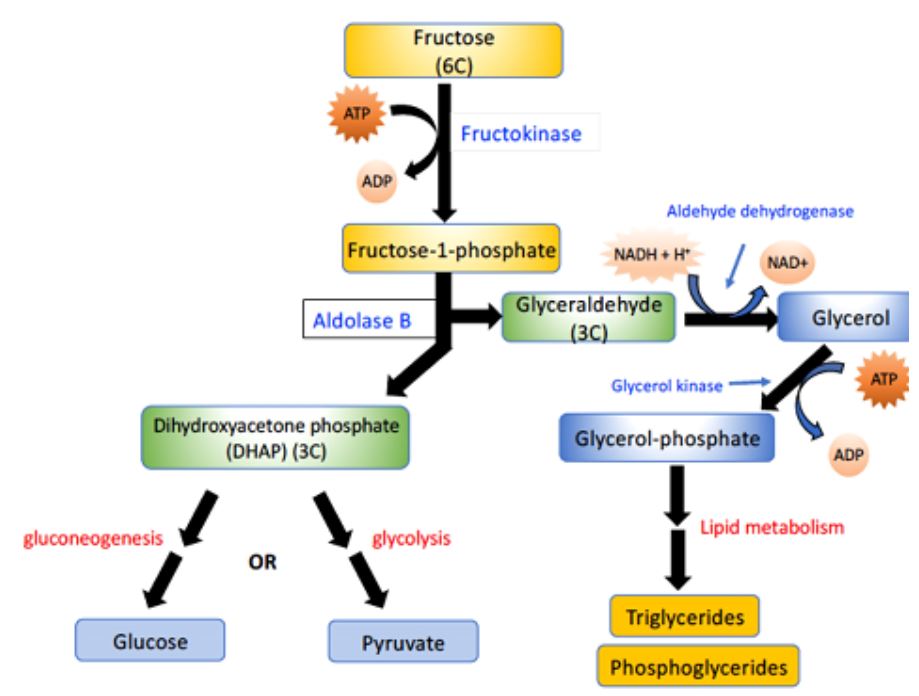
Figure 1. Overview of the metabolic fates of fructose. Fructose (top) can be converted to glucose, or pyruvate, or triglycerides.
The metabolism of fructose presents a particular challenge for the body: Fructokinase (Figure 1) is quite active and is not regulated (unlike hexokinase). The aldolase B reaction that follows is relatively slow. Therefore, on a high-fructose diet, F-1-P tends to accumulate in the liver and kidney, consuming inorganic phosphate (Pi). Pi depletion in turn creates a shortage of ATP, because Pi is required for oxidative phosphorylation. If healthy mammals are kept on a diet with fructose as the only sugar, severe liver and kidney damage can result. An extreme example of this is seen in the condition known as fructose intolerance, which results from a lack of the aldolase B enzyme that cleaves Fructose-1-phosphate. These individuals are normal, except that they become hypoglycemic and nauseous when they ingest fructose (because of inhibited liver function). People with fructose intolerance therefore need to avoid sweetened foods.
Another inherited condition involving fructose metabolism is essential fructosuria, which comes about due to a lack of fructokinase. These individuals are normal, except that they have very high levels of fructose in their urine.
SORBITOL METABOLISM
SLO2: Outline the biochemical pathways involved in metabolism of sorbitol, its intersection with glucose and fructose metabolism and consequences of sorbitol accumulation.
Conversion of glucose to fructose via the sorbitol pathway Most sugars are rapidly phosphorylated following their entry into cells. Therefore, they become trapped in the cytoplasm, because organic phosphates cannot freely cross membranes without specific
transporters. An alternate mechanism for metabolizing a monosaccharide is to convert it to a polyol (sugar alcohol) by reduction of the aldehyde group to an additional hydroxyl group.
Synthesis of sorbitol: Aldose reductase reduces glucose, producing sorbitol (Figure 2). This enzyme is found in many tissues. In cells of the liver, ovaries, and seminal vesicles, there also is a second enzyme, sorbitol dehydrogenase, which can oxidize the sorbitol to produce fructose (these cells use fructose as a major carbohydrate energy source). The pathway from sorbitol to fructose in the liver provides a mechanism by which any available sorbitol is converted into a substrate that can enter glycolysis or gluconeogenesis.
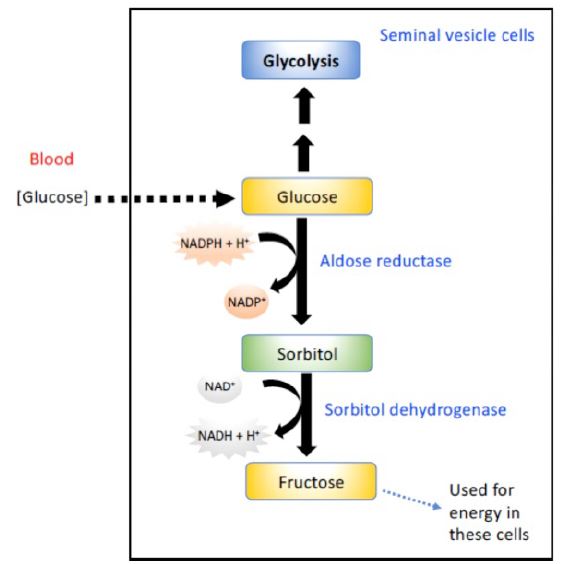
Figure 2. Conversion of glucose to sorbitol and sorbitol to fructose in cells of the seminal vesicle.
Effect of hyperglycemia on sorbitol metabolism: Because insulin is not required for the entry of glucose into the cells listed above, large amounts of glucose may enter these cells during times of hyperglycemia (for example, in uncontrolled diabetes). Elevated intracellular glucose concentrations and an adequate supply of NADPH cause aldose reductase to produce a significant increase in the amount of sorbitol, which cannot pass efficiently through cell membranes and, in turn, remains trapped inside the cell. This is exacerbated when sorbitol dehydrogenase is low or absent (for example, in retina, lens, kidney, and nerve cells). As a result, sorbitol accumulates in these cells, causing strong osmotic effects and, therefore, cell swelling as a result of water retention. Some of the pathologic alterations associated with diabetes can be attributed, in part, to this water retention, including cataract formation, peripheral neuropathy, and microvascular problems leading to nephropathy and retinopathy.
GALACTOSE METABOLISM
SLO3: Outline the biochemical pathways involved in metabolism of galactose, its intersection with glycolysis and consequences of dietary imbalance in galactose consumption and inborn errors of metabolism related to galactose metabolism including lactose intolerance.
The major dietary source of the monosaccharide galactose is from the disaccharide lactose or “milk sugar”. The digestion of lactose takes place in the small intestine, and yields one molecule each of glucose and galactose. Like fructose, the transport of galactose into cells is not insulin dependent.
As with other sugars, the metabolism of galactose must be initiated by phosphorylation, in this case by galactokinase (Figure 3).
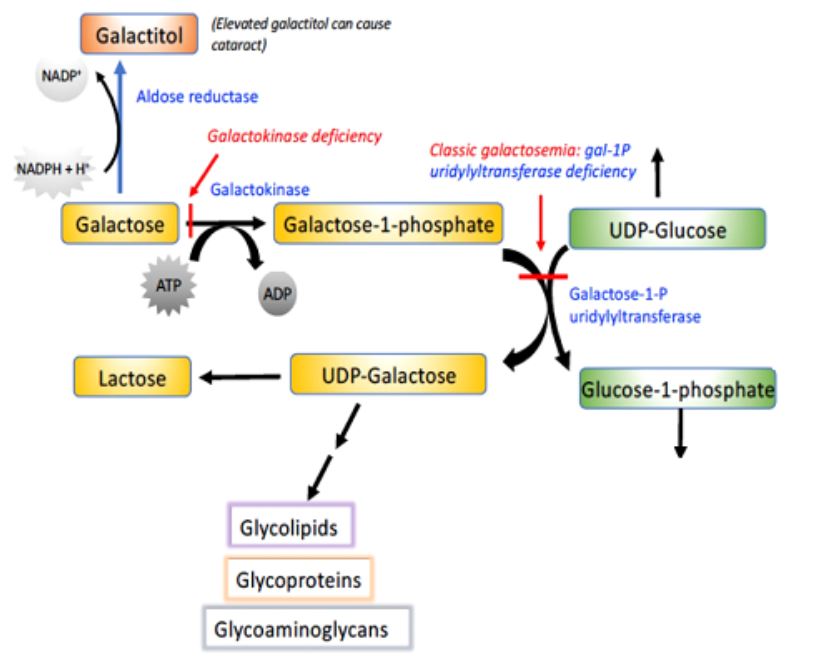
Figure 3. Overview of galactose metabolism. Galactose can be phosphorylated and then activated to be added to glycolipids,
glycoproteins, and glycoaminoglycans. When defective, several of the enzymes in this diagram lead to disease.
The resulting galactose-1-phosphate can then be activated and added to glycosylated molecules.
There is an important collection of genetic disorders in humans, known collectively as galactosemia, that are associated with this metabolic pathway. They all involve a defect in galactose metabolism, so that this sugar or its derivatives accumulate in the blood and tissues of these individuals. The most common form of galactosemia results from a deficiency in the transferase. If untreated, these individuals develop cognitive impairment, enlarged livers and cataracts. The cataracts result from the reduction of galactose to its corresponding alcohol, galactitol. If detected early, galactosemia can be treated by placing the baby on a special diet lacking
lactose.
ETHANOL METABOLISM
SLO4: Outline the biochemical pathways involved in metabolism of ethanol, its intersection with the TCA cycle and damage that can be associated with ethanol metabolism.
Ethanol is metabolized in the liver by two consecutive oxidation reactions (Figure 4). Ethanol is first converted to acetaldehyde through the action of alcohol dehydrogenase, and then acetaldehyde is oxidized to acetic acid by aldehyde dehydrogenase. Note that both of these reactions convert NAD+ to NADH.

Figure 4. Ethanol metabolism and the site of action of Antabuse (disulfiram).
Alcohol metabolism shifts the redox balance of the cell by increasing NADH relative to NAD+. Therefore, consumption of even a moderate quantity of alcohol can shift the redox balance in the cytosol of liver cells. For example, this increase in liver NADH results in elevation of blood lactic acid levels, which can cause problems in individuals with gout (discussed later in the course). Acetaldehyde is a toxic compound that normally does not normally accumulate to high levels. It is thought to contribute to 1) the effects of a hangover after drinking, 2) liver damage from chronic high alcohol consumption and 3) fetal alcohol syndrome. Antabuse (disulfiram) acts by inhibiting aldehyde dehydrogenase, thus causing an adverse reaction to alcohol consumption because of acetaldehyde buildup.
Two compounds closely related to ethanol, methanol and ethylene glycol, are quite toxic because of their conversion to corresponding aldehydes. Both alcohols are weak substrates for alcohol dehydrogenase and are converted into aldehydes that damage many tissues, including the eyes. Ethanol competes quite favorably with methanol and ethylene glycol for binding to alcohol dehydrogenase. Therefore, one therapy for poisoning by these alcohols is to continuously infuse ethanol into the patient, controlling the concentration in the bloodstream so that it effectively competes with methanol or ethylene glycol for the enzyme. This slows the formation of the toxic aldehydes and allows time for the alcohols to be excreted harmlessly in the urine.
Alcohol-related hypoglycemia
The ethanol-mediated increase in NADH causes pyruvate, oxaloacetate (OAA), and other intermediates of gluconeogenesis to be diverted into alternate pathways, resulting in the decreased synthesis of glucose. This can precipitate hypoglycemia, particularly in individuals who have depleted their stores of liver glycogen. Hypoglycemia can produce many of the behaviors associated with alcohol intoxication, such as agitation, impaired judgment, and combativeness. Therefore, alcohol consumption in vulnerable individuals (such as those who have fasted or have engaged in prolonged, strenuous exercise) can produce hypoglycemia that may contribute to the behavioral effects of alcohol. It can also increase the risk for hypoglycemia in patients using insulin.
Practice questions on fructose, galactose, and ethanol:
1. What is the significance of fructose uptake and catabolism through the Fructokinase/aldolase B pathway not being regulated by insulin?
2. Which regulatory step of glycolysis is bypassed when fructose is catabolized through Fructokinase/aldolase B? Why is this important?
3. What is the impact of excess NADH production following alcohol (ethanol) consumption?
4. Which of the following 2 deficiencies would you expect to be more severe for an individual: Galactokinase deficiency or a deficiency in the galactose-1-P uridylyl transferase enzyme? Explain your rationale.
5. How does the polyol pathway play a role in the etiology of Type 2 Diabetes?
6. What are the consequences of NADPH depletion upon production of excess sugar alcohols?
Feedback
