4 Gluconeogenesis, Glycogenesis, Glycogenolysis
Session Learning Objectives:
We start by reminding ourselves of glucose metabolism drawn as a block diagram (Figure 1). We have learned the green glycolysis pathway from glucose to pyruvate making a little ATP, the blue pentose phosphate pathway generating reducing equivalents and various sugars, the orange TCA cycle generating many reducing equivalents and several metabolic building blocks, and finally the grey electron transport chain generating ATP. In this session we will study the purple gluconeogenesis pathway for generating glucose and the yellow glycogenesis pathway for generating glycogen.
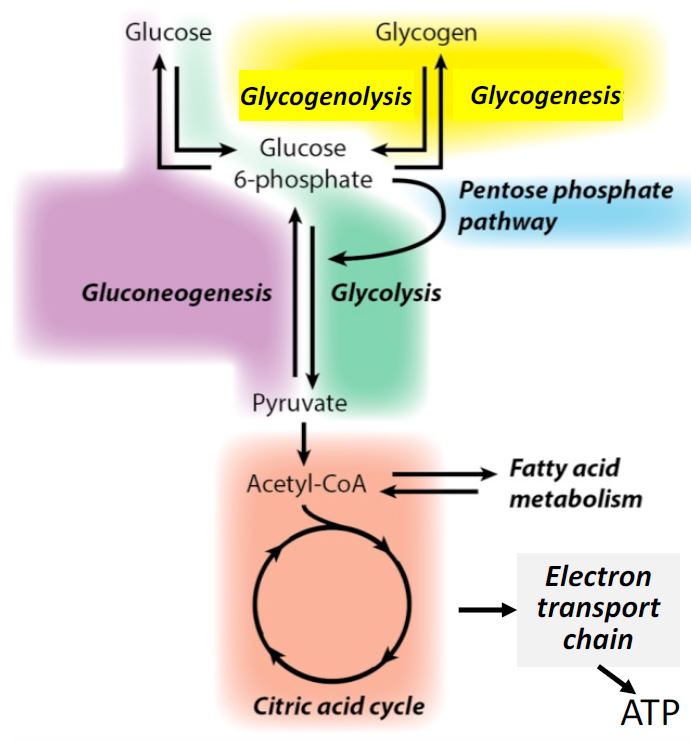
Figure 1. Block diagram of glucose metabolism. Each colored block represents a sequence of
enzymatic reactions grouped together to simplify metabolism as major functional units.
SLO1. Differentiate gluconeogenesis from glycolysis, outline 3 bypass reactions that make it energetically favorable, and explain the significance of acetyl-CoA not being a substrate.
Gluconeogenesis is the process of synthesizing glucose de novo from 3- and 4-carbon precursors such as pyruvate, alanine, or glycerol. It is an anabolic pathway. In some ways, gluconeogenesis is very similar to the reverse process of glycolysis (which is the breakdown or catabolism of glucose). However, gluconeogenesis cannot be exactly the reverse of glycolysis for two reasons: (1) it needs to be reciprocally regulated such that when glycolysis is stimulated, gluconeogenesis is turned down and vice versa; and (2) it needs to be arranged as an energetically favorable process. We have learned that glycolysis is an energetically favorable process from a thermodynamic point of view (DG < 0). Now if gluconeogenesis were exactly the reverse of glycolysis, then gluconeogenesis would be highly unfavorable (DG > 0). What we will see is that gluconeogenesis uses 7 of the 10 reactions of glycolysis but has 3 reaction steps that are specific to gluconeogenesis and that make it energetically favorable. We will call these steps “bypasses.”
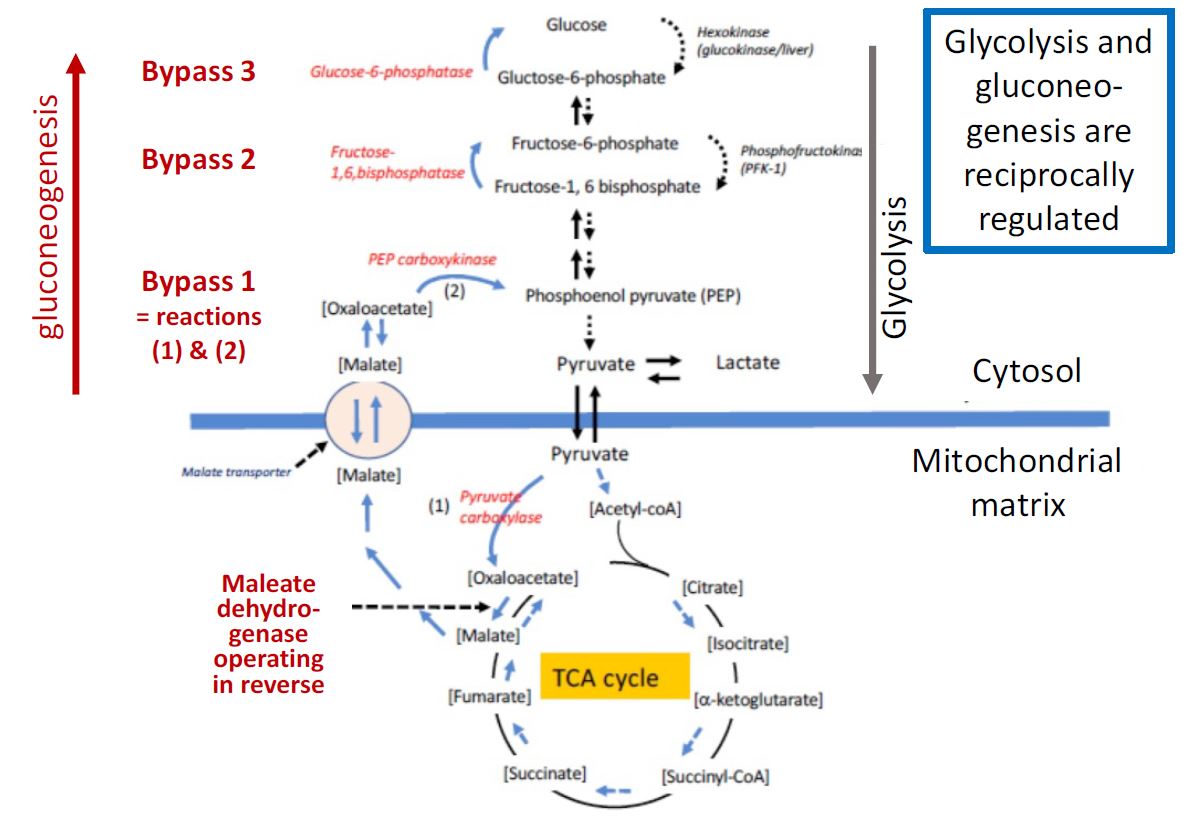
Figure 2. The relationship of liver anabolic gluconeogenesis (left half, red reactions) to the pathway of catabolic glycolysis in the cytoplasm (right half, gray arrow) and the TCA cycle in mitochondria. Bypass reactions listed in red give gluconeogenesis its anabolic direction.
The pathway of gluconeogenesis, from lactate to glucose is shown in Figure 2. Many of the reversible reactions of glycolysis are shared in common between the two pathways. However, three essentially irreversible steps of glycolysis, those catalyzed by pyruvate kinase, phosphofructokinase, and hexokinase are bypassed using different enzymes in gluconeogenesis. The liver, and to a lesser extent the kidney cortex, are capable of converting lactate and a variety of other 3-and 4-carbon molecules to glucose and further to glycogen, as will be discussed next. Gluconeogenesis is important not only in regenerating glucose from the lactate produced by exercising muscle (the “Cori cycle” see below) but also is critical in the maintenance of blood glucose levels (discussed later in the course). Note that acetyl-CoA, the product of fatty acid oxidation is NOT a substrate for gluconeogenesis. Fatty acid oxidation can be used to generate energy (ATP) for the energy-demanding process of gluconeogenesis, but its metabolic product, acetyl-CoA cannot be used as the carbon precursor. Glycerol on the other hand, which is produced from the breakdown of triglycerides into glycerol + fatty acids can be used as a substrate for gluconeogenesis (it enters the gluconeogenesis pathway at the reversible
dihydroxyacetone (DHAP)-glyceraldehyde-3-phosphate step).
A diversion on high-energy bonds and favorable reactions
This note is to help you make mechanistic sense of many reactions and pathways that you have already encountered and will encounter in your study of biochemistry. A fundamental principle in the organization of a biochemical pathway is that to be used by the cell, it has to be “spontaneous” or energetically favorable. As a reminder, in the language of thermodynamics, this concept is expressed by the statement that the ΔG should be negative. Biochemical pathways get some of their complexity just to satisfy the need to gather the energy to make them go forward. They do this by coupling a reaction that is quite favorable to a needed reaction that is not. An example is the phosphorylation of glucose by hexokinase. Addition of a phosphate to a hydroxyl functional group is energetically unfavorable. Indeed, formation of any ester or ether linkage or a peptide bond is energetically unfavorable. Hexokinase makes the glucose phosphorylation reaction go forward by coupling it to cleavage of ATP. We say that ATP has a high-energy bond since cleavage of ATP by water (called hydrolysis) would release a lot of energy and is highly favored thermodynamically. Examples of other energetically highly favored hydrolytic cleavages would be cleavage of Acetyl-CoA and cleavage of UDP-glucose, a molecule we will hear about shortly. They too have a high-energy bond which instead of being wasted, can be coupled to enzymatic reactions to push the reactions forward. This energy allows Acetyl-CoA to transfer acetyl- (2-carbons) to oxaloacetate in the TCA cycle and it allows UDPglucose to transfer glucose making linkages with other glucose moieties. It is useful to think about ATP, Acetyl-CoA, and UDP glucose as representing “activated phosphate,” “activated acetyl,” and “activated glucose,” respectively in the sense that they can transfer phosphate, acetyl, or glucose to other substrates. Much of metabolism involves using energy to make these high energy bonds and then using them. Now we
can discuss the bypass reactions of gluconeogenesis in these terms.
The bypass reactions of gluconeogenesis
Bypass step 1 of gluconeogenesis is actually a series of steps that bypass the pyruvate kinase step of glycolysis:
Half of these reactions take place in the mitochondria, and the 2nd half takes place in the cytoplasm. The pyruvate kinase step is bypassed in a complicated series of reactions that involve moving molecules into and out of the mitochondrion. Lactate is first converted to pyruvate and after transport into the mitochondrion pyruvate is converted to oxaloacetate by the reaction catalyzed by pyruvate carboxylase as follows:
Pyruvate + CO2 + ATP -> Oxaloacetate + ADP +Pi
This enzyme requires the vitamin biotin. Since there is no transporter for oxaloacetate, it is converted to malate, which is then transported out to the cytoplasm. In the cytoplasm, the malate is reconverted to oxaloacetate, which is then transformed to phosphoenolpyruvate (PEP) through the PEP carboxykinase reaction as follows:
Oxaloacetate + GTP -> PEP + GDP + CO2
These two reactions are driven by consumption of the high energy bond of ATP or GTP, respectively.
Bypass reaction 2 bypasses the phosphofructokinase (PFK) reaction of glycolysis.
In gluconeogenesis, fructose-1,6- bisphosphate is hydrolyzed to fructose-6-phosphate by the enzyme fructose-1,6-bisphosphatase (FBPase) as follows:
Fructose-1-6-bisphophate + H2O -> Fructose-6-phosphate + Pi
In glycolysis, the PFK reaction is driven in the downward catabolic direction in Figure 2 by consumption of ATP, whereas in gluconeogenesis FBPase is driven in the upward anabolic direction by cleavage of the 1- phosphate. Once fructose-6-phosphate is formed, it is easily converted to glucose-6-phosphate using the reversible glucose-6-P isomerase reaction.
Bypass reaction 3 bypasses the hexokinase/glucokinase reaction of glycolysis.
In the liver, once glucose-6P is formed, it can be converted to glucose by the enzyme glucose-6-phosphatase. This enzyme plays a critical role in the function of the liver in regulating blood glucose levels (discussed in more detail later on). As with bypass 2, in glycolysis the catabolic conversion is driven downward in Figure 2 by consumption of ATP, and in gluconeogenesis, the anabolic bypass 3 is driven upward by cleavage of a phosphate.
Session Learning Objective 2. Diagram the mechanisms by which glucose synthesis and glucose breakdown are reciprocally regulated. Key questions to keep in mind are “when is gluconeogenesis important? Which tissues are most affected by this process?”
Regulation of gluconeogenesis. Gluconeogenesis and glycolysis share many of the same reactions and regulation is clearly required to prevent futile cycles where one pathway is continually undoing the results of the other. This regulation is achieved in two ways. First, gluconeogenesis and glycolysis are reciprocally regulated by the hormones glucagon and insulin as part of the mechanism for controlling blood glucose levels. Second, regulation of gluconeogenesis depends on the energy charge of the cell in a manner that is exactly opposite from regulation of glycolysis, so that when glycolysis is on, gluconeogenesis is off, and vice versa. The interconversion of fructose-6-phosphate and fructose-1,6-bisphosphate is highly regulated. As discussed in the chapter on glycolysis, PFK-1 is stimulated by AMP and inhibited by ATP, therefore, this step in glycolysis is activated by low energy charge and inhibited by high energy charge. In contrast, the reverse reaction, catalyzed by FBPase, is inhibited by AMP. Thus, at low energy charge, when PFK-1 is turned on, FBPase is turned off. Conversely, when PFK-1 is turned off by high energy charge, AMP is low which allows FBPase to be active.
The interconversion of phosphoenolpyruvate and pyruvate is also precisely controlled. Recall that the pyruvate kinase reaction of glycolysis is also regulated by energy charge. Pyruvate carboxylase, which catalyzes the first step in the conversion of pyruvate to glucose, is activated by acetyl CoA. Elevated acetyl CoA signals the need for more oxaloacetate. When energy charge is high, the TCA cycle is turned off and oxaloacetate flows
in the direction of gluconeogenesis. When energy charge is low, oxaloacetate enters into the TCA cycle by condensing with acetyl CoA, to form citrate.
In the liver, several hormones interact to regulate the transcription of the PEP carboxykinase gene. Expression of this gene is stimulated by glucocorticoids and glucagon, both of which stimulate gluconeogenesis. Insulin has the converse effect on transcription of the PEP carboxykinase gene.
Session Learning Objective 3. Understand how gluconeogenesis in liver helps maintain anaerobic glycolysis in active skeletal muscle through the Cori cycle.
The Cori Cycle: The Cori cycle refers to a process in which lactate derived from glucose in skeletal muscle is delivered to the liver, converted back to glucose, and returned to muscle via the blood. Much of the lactate produced in a 24 hr period is recycled in this manner. The Cori cycle
(Figure 3) illustrates well the way two organs can work together to achieve a biochemical objective. Lactate is produced from glucose (derived from glycogen) by glycolysis in anaerobic muscle and is transported to the liver via the blood stream. In the liver, lactate is converted back again to glucose through gluconeogenesis. The energy for synthesis of glucose in the liver comes from the oxidation of fatty acids.
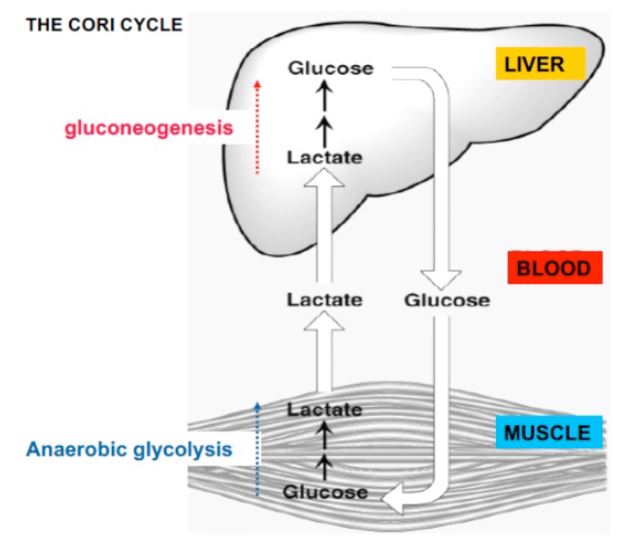
Figure 3. In the Cori cycle glucose is converted to lactate in active muscle, delivered to the liver by the blood stream, converted back to glucose, and then returned to the muscle though the blood stream.
Some medical consequences: The rate of gluconeogenesis is strongly controlled by circulating levels of glucagon, insulin and cortisol. Conditions characterized by imbalances in these hormones can either cause hypoglycemia (insulinomas, ethanol ingestion), or an accelerated gluconeogenesis and accompanying hyperglycemia (diabetes, Cushing syndrome). Van Gierke’s disease results from a deficiency in glucose 6-phosphatase, an enzyme of gluconeogenesis. Chronic hyperglycemia leads to degrees of diabetic retinopathy, nephropathy, neuropathy, and sugar cataracts in type 2 diabetes.
Practice questions on gluconeogenesis:
1. What are the key biochemical features of the regulated steps of gluconeogenesis?
2. What is the primary function of gluconeogenesis in the liver?
3. How is reciprocal regulation of glycolysis and gluconeogenesis ensured?
4. What would you expect in a patient who has a deficiency in glucose-6-phosphatase?
5. Why does gluconeogenesis play such a critical role in maintaining blood glucose level homeostasis?
GLYCOGEN METABOLISM
Session Learning Objective 4. Diagram glycogen as a branched polymer. Contrast the use of glycogen in liver and muscle. Understand the pathways by which glycogen is synthesized and broken down.
Glycogen metabolism overview:
Glycogen is the main storage form of glucose, and is key to mobilizing glucose stores in skeletal muscles during vigorous exercise. Glucose molecules are osmotically active particles, thus they cannot be stored in high concentration as monomers. Linking glucose molecules as an enormous polymer such as glycogen circumvents the osmotic pressure issue. The main stores of glycogen are found in skeletal muscle and liver, although most other cells store small amounts of glycogen for their own use. The function of muscle glycogen is to serve as a fuel reserve for the synthesis of ATP during muscle contraction (Figure 4). That of liver glycogen is to maintain the blood glucose concentration, particularly during the early stages of a fast.
Glycogenesis, the process of glycogen anabolism:
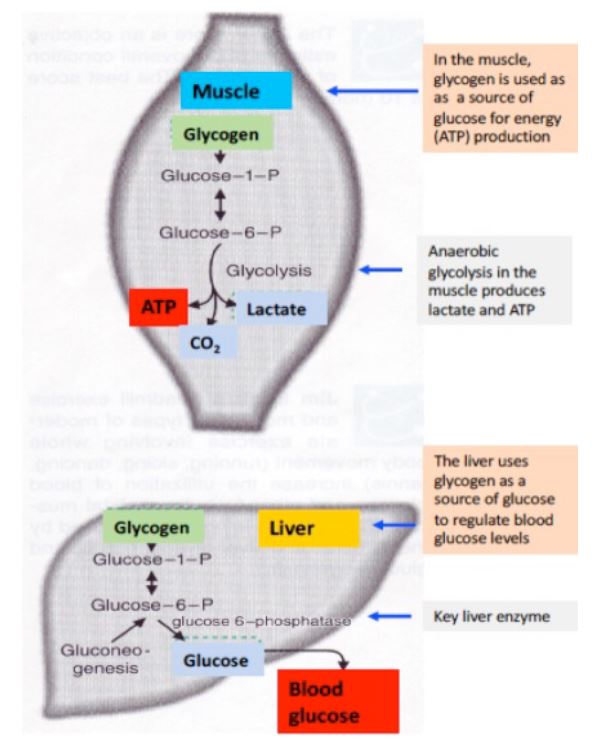
Figure 4. Glycogen stores serve different roles in muscle and liver. –>
Structure of glycogen: Glycogen is a branched-chain polysaccharide made from α-D-glucose. The glucose monomers link to form a primary linear polymer, and after an average of eight to ten glucosyl residues, there is a branch formed by a different glycosidic linkage. Figure 5 diagrams the resulting branched polymer that is connected to a protein at the center. Glucose is added and removed from the many “nonreducing” ends, so there are hundreds of ends available for the reactions of synthesis and breakdown.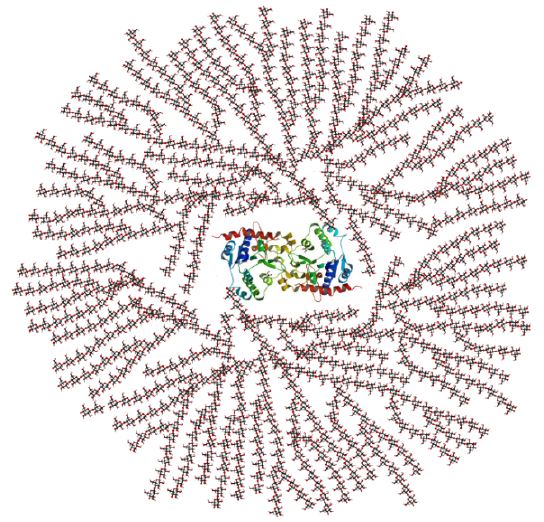
Figure 5. ^ Glycogen is made from α-D glucose. The primary glycosidic bond is an α(1→4) linkage. After an average of eight to ten glucosyl residues, there is a branch containing an α(1→6) linkage. The concept of a branched polymer is important for you to visualize; the chemistry is not.
Glycogenolysis, the process of glycogen catabolism:
During vigorous exercise, the delivery of both oxygen and glucose to skeletal muscle through the blood stream cannot keep up with the demand from the aerobic metabolism of glucose or of fatty acids. Therefore, under these anaerobic conditions muscle becomes dependent on the stored glucose in glycogen, to generate ATP. The breakdown of glycogen is complicated in that the glycosidic linkages of both the linear parts and the branches need to be broken in order to achieve complete release of glucose residues. Therefore, this process requires two enzymes, glycogen phosphorylase and debranching enzyme.
The enzyme phosphoglucomutase then converts glucose-1-phosphate to glucose-6-phosphate for entry into glycolysis, if needed. Note that one less ATP per glucose molecule is required to enter glycolysis from glycogen, compared to starting with free glucose. Anaerobic glycolysis beginning with glycogen therefore yields a net production of 3 ATP per glucose.
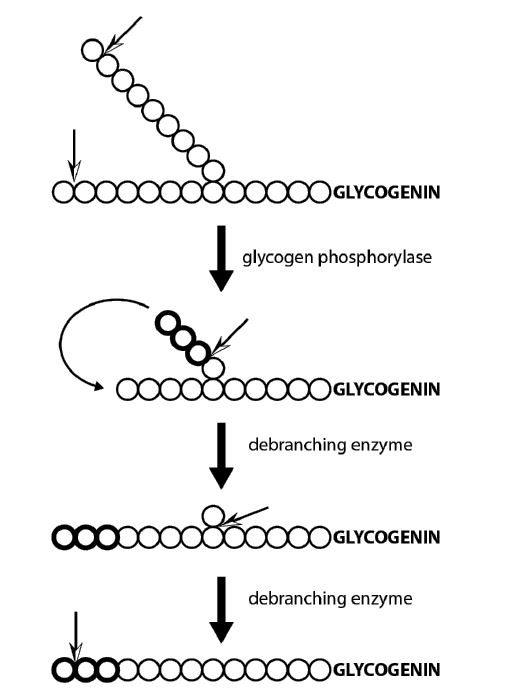
Figure 6. The combined activities of glycogen phosphorylase and the debranching enzyme break down a glycogen molecule. When glycogen phosphorylase get close to a branch point, a debranching enzyme resolves the branch structure and glycogen phosphorylase continues.
Regulation of glycogen phosphorylase in striated muscle. Glycogen phosphorylase is the key regulated enzyme in glycogen breakdown in muscle. Its activity is responsive to: 1) the energy charge of a muscle cell, 2) the contractile state of the muscle and 3) the circulating levels of the hormone epinephrine.
Glycogen phosphorylase can be activated by phosphorylation. In muscle, the phosphorylation state of this enzyme is regulated by at least two signals (Figure 7). Muscle contraction is triggered by an elevation of intracellular Ca2+. This elevated calcium also stimulates phosphorylation of
glycogen phosphorylase through calcium binding proteins that interact with phosphorylase kinase and partially activate it. Glucose-1-phosphate, is then released from glycogen reserves by the activated phosphorylase and provides energy for contraction. In addition, the phosphorylation of glycogen phosphorylase is stimulated by the “fight or flight” hormone epinephrine in a signaling cascade. Epinephrine from the blood stream binds to beta adrenergic receptors and activates adenylate cyclase, which produces cyclic AMP (cAMP). cAMP, in turn, activates protein kinase A (PKA). PKA then phosphorylates phosphorylase kinase, converting it from the inactive to the active form. The active phosphorylase kinase subsequently phosphorylates inactive phosphorylase, converting it into the active form and thereby stimulating glycogen breakdown. You might wonder why cAMP doesn’t activate phosphorylase directly. The extra steps used allow amplification of the hormonal signal and provide additional regulation points. We will discuss the hormonal regulation of glycogen phosphorylase in the liver in more detail later, in the context of its reciprocal regulation with glycogen synthase.
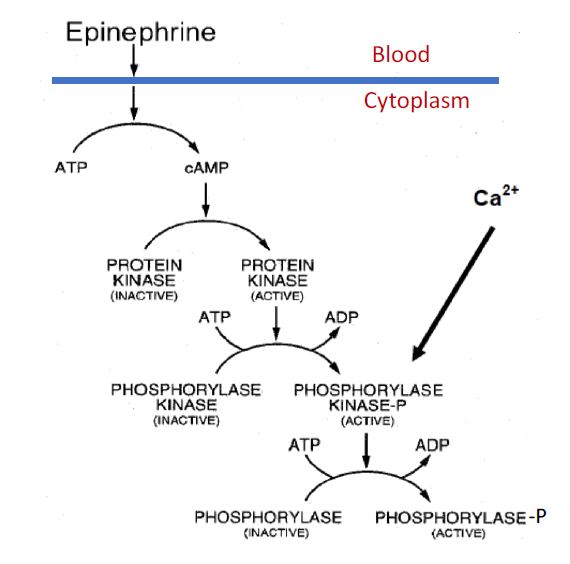
Figure 7. Regulation of muscle glycogen phosphorylase by protein phosphorylation and dephosphorylation.
Glycogen Synthesis:
In resting muscle after exercise, glycogen is resynthesized using glucose delivered to the muscle via the bloodstream. The key enzyme is glycogen synthase, which extends glycogen chains by one glucose molecule at a time. The glycogen synthase enzyme uses an activated form of glucose called uridine-diphosphate (UDP)- glucose.
The high energy UDP-glucose is formed enzymatically by reaction of glucose-1-phosphate with UTP. In addition, synthesis requires a mechanism to make glycogen branchpoints. Introduction of α-1,6 linkages is catalyzed by the enzyme called “branching enzyme.” This reaction creates two ends, both of which can be extended by glycogen synthase (Figure 8 V).
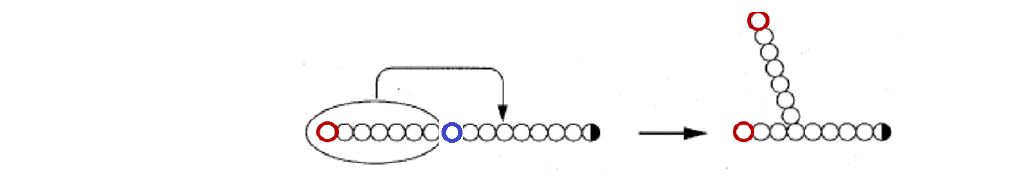
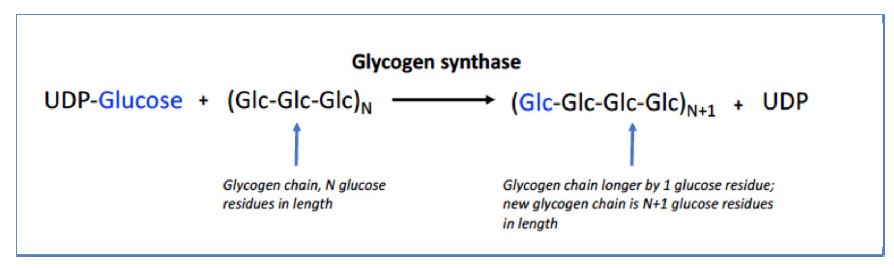
Figure 8. Branching enzyme introduces a branch in an existing linear nonreducing end of glycogen (terminating in the left red glucose residue) by cutting at the blue residue and pasting the cut segment further back in 1-6 glycosidic linkage. This process generates two nonreducing ends (red residues).
Key take home points: Glycogen is the body’s way of storing its reserve of glucose as a giant polymer. Glycogen breakdown requires alternating processing by the enzymes glycogen phosphorylase and debranching enzyme. Glycogen synthesis requires alternating processing by the enzymes glycogen synthase and branching enzyme.
Session Learning Objective 5. Diagram the mechanisms by which glycogen synthesis and glycogen breakdown are reciprocally regulated.
Glycogen metabolism – interplay between catabolism (degradation) and synthesis: We have just described glycogen synthesis from glucose-1P and glycogen breakdown to glucose-1P. These steps are encapsulated in reactions 4 &5 and reaction 6, respectively, of Figure 9. Reactions 1, 2 ,
and 3 remind us of the relationship of glucose-1P to glucose and to glycolysis in the liver. Reactions 1, 3, 4 and 5 are necessary to replenish glycogen stores, using blood glucose as a source. Reaction 6 is the glycogen phosphorylase reaction that breaks down glycogen. In the liver, it is coupled to reactions 3 and 2, producing glucose for release into the blood when plasma glucose levels need to be maintained.
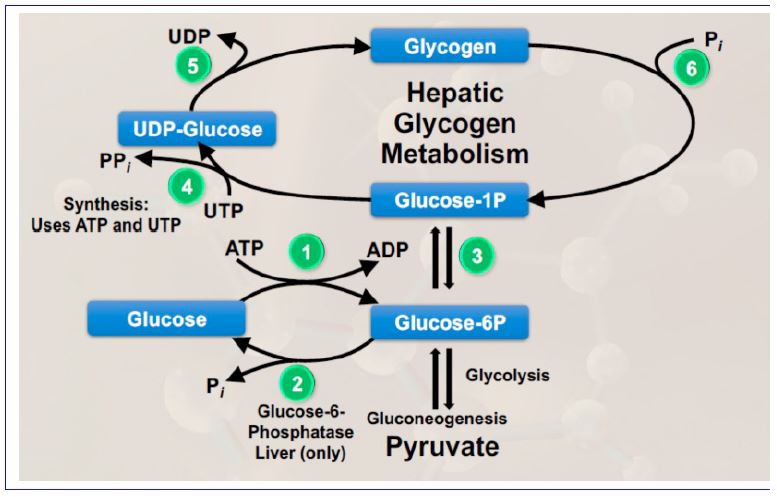
Figure 9. Summary of glycogen metabolism in the liver and its relationship to glucose and glycolysis.
If reactions 4, 5 and 6 are allowed to run in an uncontrolled manner, a “futile cycle” would be formed in which high energy phosphates would be removed from UTP for no productive purpose. As a general rule in metabolism, futile cycles are prevented through reciprocal regulation of catabolic and anabolic processes, our next subject.
Regulation of hepatic glycogen synthase (GS): Like glycogen phosphorylase, glycogen synthase is present in 2 forms (Figure 10), but here, the active form is unphosphorylated, and the inactive form is phosphorylated. This is exactly the opposite from glycogen phosphorylase. To remember which form is active, keep in mind that the hormonal signal to release glucose is epinephrine, which activates pathways that lead to phosphorylation of both proteins. The opposite effects of phosphorylation of these enzymes ensures that if glycogen degradation is up-regulated, glycogen
synthesis is down regulated, and vice versa. We will discuss the regulation of hepatic glycogen synthase and glycogen phosphorylase within the context of the feed-fast cycle and the relative levels of insulin to glucagon in another chapter.
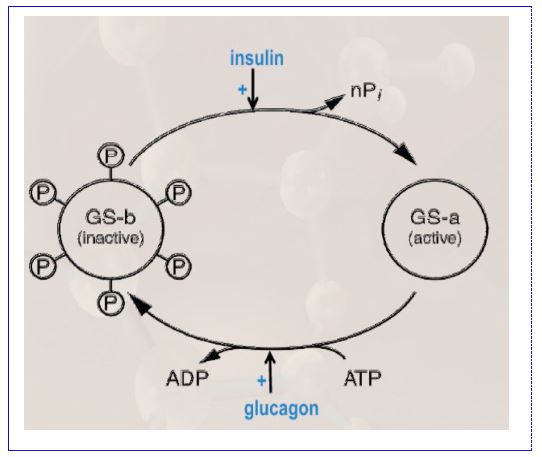
Figure 10. Reciprocal hormonal regulation of glycogen synthase (GS). Glucagon inactivates GS by stimulating its phosphorylation. Insulin activates GS by stimulating its dephosphorylation.
Session Learning Objective 6. Outline genetic disorders of glucose mobilization (Von Gierke, Pompe, Cori, Andersen, McArdle) including clinical manifestations, lab values and biochemistry.
All inborn error of metabolism disorders of glucose mobilization are rare.
Andersen: defect in glycogen branching enzyme. Glycogen has long chains with few branches form inclusions. Hypoglycemia, cirrhosis, death within ~5 years.
Cori: defect in debranching enzyme. Glycogen has short outer branches. Mild hypoglycemia and hepatomegaly.
McArdle: defect in muscle glycogen phosphorylase. Normal glycogen, muscle cramps and weakness, often manifests in teenage years.
Pompe: defect in lysosomal a1,4 glucosidase. Cardiomegaly, weakness, death.
Von Gierke: defect in glucose 6 phosphatase. Sever hypoglycemia, hepatomegaly, lactic acidosis.
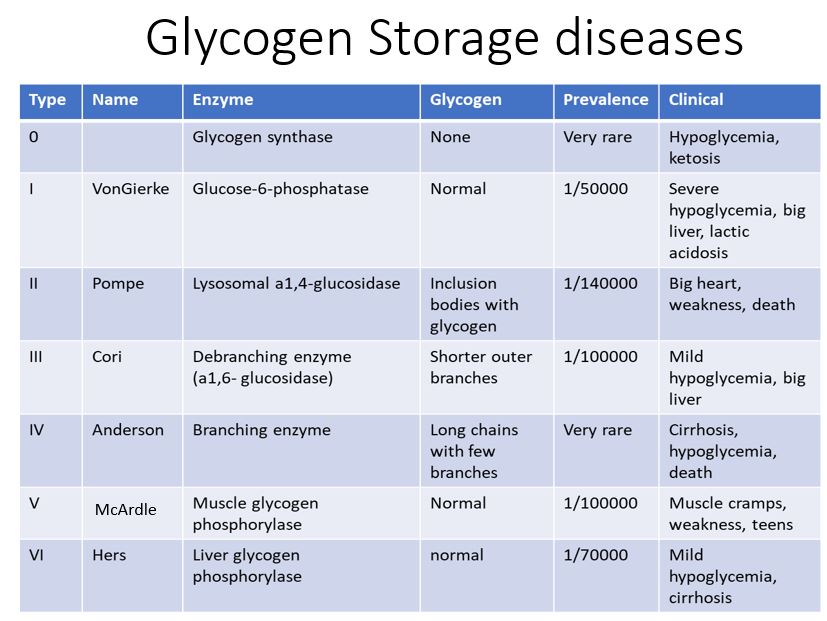
Figure 11. Clinical correlates of glycogen storage disorders.
Practice questions on glycogen metabolism:
1. What is the biological role of glycogen in striated muscle? How does this differ from its role in the liver?
2. Under low insulin to glucagon ratio conditions in the liver, which enzyme would you expect to be activated, glycogen synthase or glycogen phosphorylase? Can you explain your reasoning? (hint: what are blood glucose levels like when insulin is produced versus when glucagon is produced?)
3. What would you expect in a patient who has a deficiency in debranching enzyme?
4. Why is it important for phosphorylation to have an opposite effect on glycogen synthase compared to its effect on glycogen phosphorylase?
5. Would you expect a person who is not able to synthesize glycogen to be able to survive? Why or why not?
Feedback
