15 Purine, Pyrimidine Metabolism, Disorders
Session Learning Objectives:
SLO 1. Identify the key points pertaining to the de novo purine and pyrimidine biosynthesis pathways, emphasizing input metabolites, key regulated steps, and significance for physiology and metabolism.
SLO 2. Examine the formation of deoxyribonucleotides and the balancing of nucleotide pools.
SLO 3. Summarize the key aspects of thymidine biosynthesis and describe the mechanism by which inhibitors of this pathway act in cancer chemotherapy.
SLO 4. Analyze the catabolism of nucleotides and the purine salvage pathway, focusing on its role in the pathophysiology of gout.
Overview: This session covers the metabolism of nucleotides and covers the following primary topics:
- The structures and nomenclature of nucleotides and their constituent parts.
- De novo synthesis of purines on PRPP
- De novo synthesis of pyrimidines and conjugations to PRPP
- Reduction of nucleotides to deoxynucleotides
- Recycling of purine bases
- Degradation of purines to uric acid
As you read through the course packet and work through the SLOs make note of genetic disorders and drugs to treat disorders of nucleotide metabolism or to inhibit nucleotide metabolism as a treatment for cancer and other conditions.
SLO 1. Identify the key points pertaining to the de novo purine and pyrimidine biosynthesis pathways, emphasizing input metabolites, key regulated steps, and significance for physiology and metabolism.
Purine and Pyrimidine Review
Nucleotides are used as substrates in the biosynthesis of the nucleic acids RNA and DNA, in regulation of enzymatic reactions, and as a source of energy. The bases within nucleotides are either purines or pyrimidines, which are heterocyclic compounds whose rings contain carbon and nitrogen. The planar character of purines and pyrimidines facilitates their close association or stacking, which stabilizes double stranded DNA.
Guanine, adenine and cytosine are used in RNA and DNA while thymine is used in DNA and uracil in RNA. Sometimes uracil can appear in DNA as an error that is removed during DNA repair. Guanine (G) base-pairs with cytosine (C) and adenine (A) base-pairs with thymine (T) in DNA and with uracil (U) in RNA.
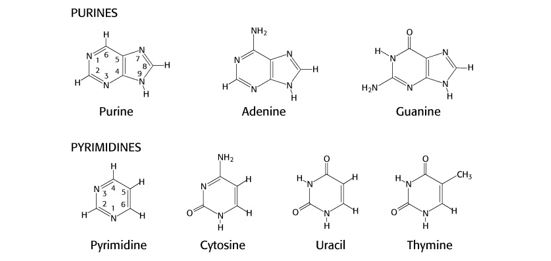
Figure 1.
It is not necessary to memorize the exact structures or atom numbering of these nitrogenous bases. However, you should be aware of:
- the use of base analogs that can be incorporated into DNA and used experimentally in the lab or therapeutically as anti-cancer drugs, such as 8-azaguanine (N at position 8 in the ring instead of C), 6-thioguanine (S bound to C6 instead of O) or 5-fluorouracil (F bound to C5 instead of H)
- processes that modify bases, such as methylation of cytosine to 5-methyl-C or the deamination of adenine to inosine or cytosine to uracil. The biological consequences of these base-modifying processes are discussed in other sections of this course.
Nucleosides and Nucleotides
When a purine or pyrimidine base is attached to a ribose, it is called a nucleoside. The addition of a phosphate to the ribose changes its designation to nucleotide. Nucleosides and nucleotides have different names listed in the table below, but in common usage the names are often used inaccurately and interchangeably, and the shortcut is to use the 1-letter designation.
A deoxyribonucleotide is a ribonucleotide reduced at the 2’ position of the ribose and is designated with a “d” in front of the name. An exception to this convention is thymidine, which is a deoxyribonucleoside without the “deoxygenated” prefix in its name. Thymidylate (TMP) is a deoxyribonucleotide, but is often written as “dTMP” for clarity. The structures of adenosine (a nucleoside) and deoxyadenosine monophosphate (dAMP, a deoxyribonucleotide) are shown here.

Table 1. Modified from Biochemistry, 6th edition, Freeman and Co. (2007)
Purine and pyrimidine pathways compared
The ribose for both purines and pyrimidines originates from the pentose phosphate pathway (hexose monophosphate shunt) and is used as 5-phosphoribosylpyrophosphate (PRPP) in the biosynthetic pathways for purines and pyrimidines.
Ribose 5-P + ATP <–> PRPP + AMP
Phosphoribosylpyrophosphate synthetase
De novo purine (Pu) and pyrimidine (Py) biosynthetic pathways are highly conserved in nature and proceed via two conceptually different biochemical strategies:
- The purine ring is built on PRPP.
- The pyrimidine ring is synthesized first, and PRPP is the source for the ribose 5-P added to the pyrimidine ring.
While both pathways involve several enzymatic steps, multi-functional enzymes with more than one enzymatic activity and multi-enzyme complexes in a cell promote increased efficiency of the pathways.
The final catabolite of purines in humans is uric acid that is excreted or may precipitate as sodium urate in excess. Pyrimidines are catabolized to CO2, H2O and the nitrogen is excreted via the urea cycle.
Purine biosynthesis, de novo pathway
The atoms in the purine ring originate from amino acids (aspartate, glycine and glutamine), CO2 and a folate derivative (N10-formyl-THF). Normal folate metabolism is required for synthesis of purines and formation of dTMP (a pyrimidine), needed as a precursor for dTTP used in DNA synthesis. Disruption of purine and pyrimidine biosynthetic pathways is used as a strategy for inhibiting the propagation of quickly dividing cells in cancer.
At the end of the so-called common pathway for de novo biosynthesis of purines, inosine monophosphate (IMP) is generated and acts as a precursor for the synthesis of AMP and GMP. Balance in the ribonucleotide pool is regulated in part by feedback inhibition by on the first two common steps in the de novo pathway and the dedicated reactions for synthesizing AMP or GMP. The availability of ribose 5-P and PRPP also has an effect on the functioning of this pathway. Glutamine-PRPP amidotransferase catalyzes the committed step and may be inhibited by the anti-cancer glutamine analogs azaserine and acivicin.
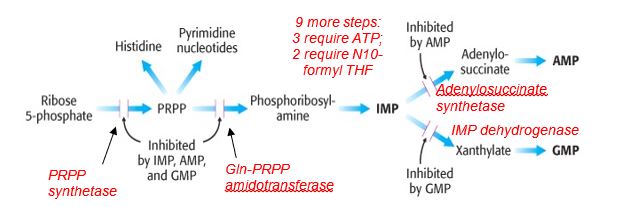
Figure 3.
Key points for de novo purine biosynthesis
- Precursors for the purine ring include amino acids and a derivative of tetrahydrofolate (THF); deficiency in folate metabolism can compromise purine biosynthesis.
- De novo purine biosynthesis is an energy-requiring process involving ATP.
- The purine ring is built on the ribose-containing substrate phosphoribosyl pyrophosphate (PRPP) synthesized from ribose 5-P, a product of the pentose phosphate pathway.
- Purine biosynthesis employs a common pathway to IMP, is activated by PRPP, and is feedback inhibited by IMP, AMP and GMP.
- AMP and GMP inhibit their own respective pathways, regulating the balance of AMP and GMP production.
Pyrimidine biosynthesis
The mammalian pyrimidine biosynthetic pathway starts with synthesis of carbamoyl phosphate by the cytosolic carbamoyl phosphate synthetase II using glutamine as a nitrogen donor. This is in contrast to the mitochondrial carbamoyl phosphate synthetase I, which uses ammonia and is part of the urea cycle. PRPP is the source of the ribose and phosphate in the synthesis of UMP. The UMP product of pyrimidine biosynthesis is the substrate for multistep processes to produce dTMP and CTP.
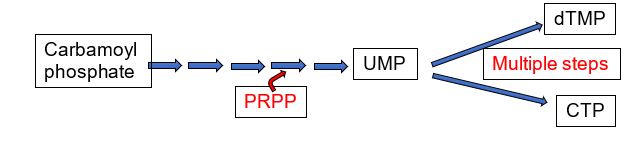
Figure 4.
In eukaryotes, carbamoyl phosphate synthetase II is part of a trifunctional enzyme (“CAD”) catalyzing the first three steps of pyrimidine biosynthesis. Intermediates remain bound to the enzyme, increasing the catalytic efficiency of the enzyme. The trifunctional enzyme is regulated in humans through inhibition by UTP and activation by PRPP. The final two steps of the common pathway leading up to the UMP product are catalyzed by a bifunctional enzyme UMP synthase, further increasing catalytic efficiency. Thus, the entire pathway of 6 enzymatic activities utilizes trifunctional, monofunctional, and bifunctional enzymes.
Genetic defects in the gene encoding the bifunctional UMP synthase will cause an excess of orotate, resulting in the disorder orotic aciduria.
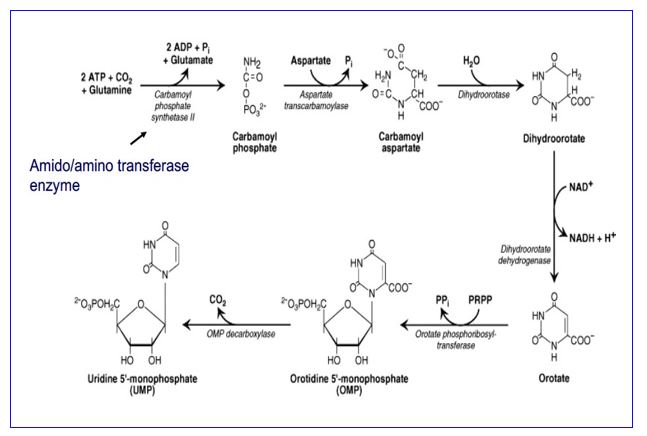
Figure 5.
The production of dTMP, as a precursor for DNA synthesis and repair, is required for cell division. Consequently, thymidylate synthase (TS), catalyzing dUMP -> dTMP, is a target for anti-cancer drugs. The thymidylate synthase reaction requires a tetrahydrofolate derivative for methylation of dUMP to form dTMP, so a deficiency or inhibition of folate metabolism can compromise production of dTMP and in turn, cell division.
Another enzyme, thymidine kinase (TK) catalyzes the phosphorylation of two deoxynucleosides, deoxyuridine or thymidine, to produce dUMP and dTMP, respectively. Viral thymidine kinase (TK) is not as fastidious as mammalian TK and is capable of using different substrates. Consequently, toxic nucleotide analogs (e.g. 8-azaguanine) can be phosphorylated and incorporated into viral DNA when viral TK is present and may be used in some anti-viral strategies. The basic pathways catalyzed by these two enzymes is shown below.
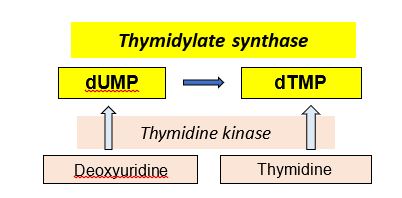
Figure 6.
Key points for pyrimidine biosynthesis
- In an energy-requiring pathway, amino acids are used in the synthesis of the pyrimidine ring, followed by the addition of the ribose from phosphoribosyl pyrophosphate (PRPP), synthesized from ribose 5-P, a product of the pentose phosphate pathway.
- Enzymatic efficiency: trifunctional enzyme (CAD) catalyzes first 3 steps and bifunctional UMP synthase, the last 2 steps.
- Common pathway until UMP production, then different multi-step pathways are required to produce CTP and dTMP
- A derivative of tetrahydrofolate is required for methylation of dUMP in the thymidylate synthase reaction catalyzing dUMP -> dTMP.
- Importance of thymidylate synthase (TS), thymidine kinase (TK)
- TS may be inhibited by an anti-cancer drug or a deficiency in folate metabolism; viral TK can use toxic base analogs that the human enzyme cannot.
SLO 2. Examine the formation of deoxyribonucleotides and the balancing of nucleotide pools.
Deoxyribonucleotides lack a hydroxyl group at the 2’ position of the ribose. Ribonucleotide reductase catalyzes reduction of a ribonucleotide diphosphate (NDP) to a deoxyribonucleotide diphosphate (dNDP). Without ribonucleotide reductase there would be no DNA!
The coenzyme thioredoxin is a reducing agent in the reaction catalyzed by ribonucleotide reductase. Reduced thioredoxin is regenerated with thioredoxin reductase, using NADPH.
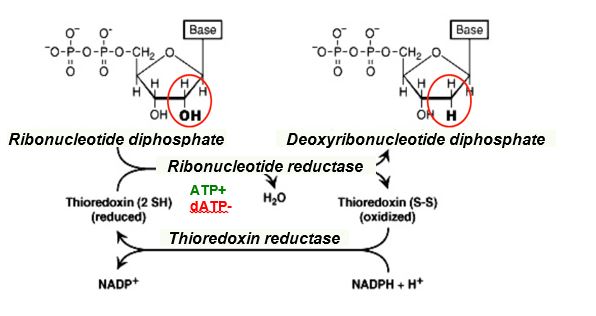
Figure 7.
Ribonucleotide reductase is regulated at two levels and is directed at maintaining balance in the nucleotide pool. The enzyme activity and substrate specificity are regulated allosterically. For example,
- Activity sites: ATP binding increases the net rate of enzymatic activity and dATP binding inhibits the overall catalytic activity
- Substrate specificity: Specific nucleotides regulate enzyme substrate specificity, allowing reduction of specific ribonucleotides and contributing to the balance of nucleotides in the cellular pool.

Figure 8.
Without balance in the nucleotide pool, the excess of some nucleotides could potentially drive incorporation of the incorrect nucleotide during DNA synthesis and result in increased errors.
SLO 3. Summarize the key aspects of thymidine biosynthesis and describe the mechanism by which inhibitors of this pathway act in cancer chemotherapy.
Thymidylate synthase (TS), with substrates dUMP and a THF derivative, catalyzes the synthesis of the deoxyribonucleotide thymidylate, also called dTMP. This reaction also yields dihydrofolate (DHF) subsequently reduced by dihydrofolate reductase (DHFR). Thus, TS and DHFR act in concert to regenerate THF, thus maintaining availability of THF for use in the folate cycle.
Figure 9.
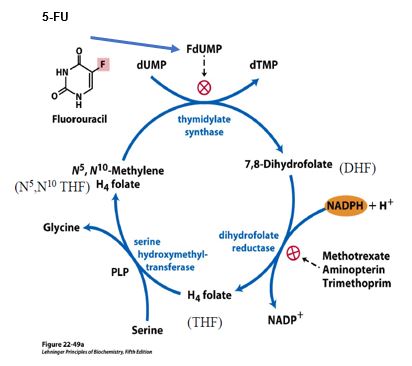 Figure 9.
Figure 9.
Intentional disruption of folate metabolism is part of several anti-cancer or anti-microbial strategies. For example, DHFR is inhibited by several drugs including methotrexate, used in therapies for cancer and rheumatoid arthritis. Trimethoprim is an antibiotic that binds bacterial DHFR with an affinity that is orders of magnitude greater than its affinity for human DHFR. Aminopterin was previously used as an anti-cancer agent but is now used predominantly as a selective agent in experimental protocols in the laboratory.
5-fluorouracil (5-FU) is a pyrimidine analog commonly used in combination chemotherapy for several different cancers. 5-FU is also formulated as a topical cream for treating skin cancer and certain pre-cancerous lesions. 5-FU is converted to Fluoro-dUMP (FdUMP), which binds irreversibly to the active site of thymidylate synthase, resulting in deficiency of dTMP, and therefore reduced dTTP for DNA synthesis.
There is significant genetic variation in the population in the DPYD gene encoding dihydropyrimidine dehydrogenase (DPD), catalyzing the first step in the degradation of 5-FU. Normally the majority of 5-FU is catabolized to its inactive form by the DPD enzyme. However, depending on the allelic variant present, a DPD deficiency or DPD enzyme that is a poor metabolizer of 5-FU can lead to severe toxicity and cause a serious adverse drug reaction that can be lethal. Diagnostic kits are available for genetic testing prior to a potential use of 5-FU.
SLO 4. Analyze the catabolism of nucleotides and the purine salvage pathway, focusing on its role in the pathophysiology of gout.
While pyrimidine salvage is not considered biochemically significant, purine salvage pathways contribute to replenishing intermediates in the purine biosynthetic pathway. Purine salvage pathways are catalyzed by phosphoribosyltransferases that recycle purine bases into the de novo purine biosynthetic pathway. Hypoxanthine-guanine phosphoribosyltransferase (HPRT or formerly called HGPRT), using PRPP as the ribose 5-P donor, recycles hypoxanthine to IMP and guanine to GMP, and adenine phosphoribosyltransferase (APRT) recycles adenine to AMP.
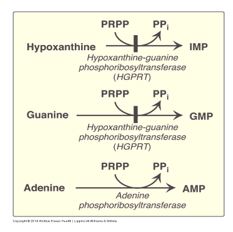
Figure 10.
A deficiency in a salvage pathway enzyme causes overproduction of purines. This counter-intuitive result is best explained by the model where a decrease in PRPP usage by a salvage pathway provides more PRPP to stimulate de novo purine biosynthesis, overriding negative feedback regulation by IMP, AMP, GMP.
X-linked genetic defects in the HPRT gene cause a spectrum of neurological symptoms. The most severe forms cause Lesch Nyhan syndrome, associated with excess production of purines and uric acid, resulting in gout. Neurological symptoms may include intellectual disability and self-injurious behavior (self-mutilation), managed with physical restraints as well as pharmaceutical interventions. A partial deficiency of HPRT may cause a Lesch Nyhan variant syndrome where patients present with hyperuricemia and gout but milder neurological manifestations.
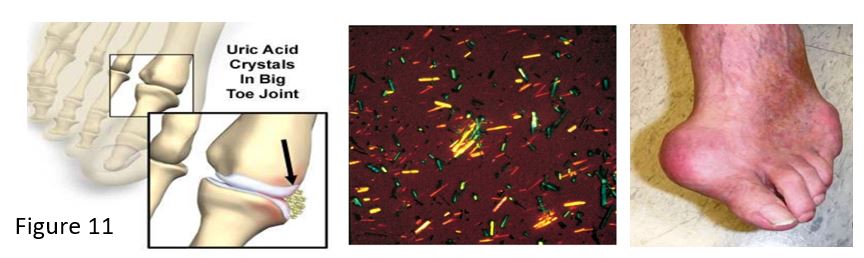
Gout may result from overproduction or underexcretion of uric acid. In Lesch Nyhan syndrome or other situations causing elevated uric acid, such as from increased cell lysis during trauma or chemotherapy, sodium urate crystals (tophi) deposit in a joint and cause an inflammatory reaction. White blood cells phagocytose the tophi and crystals disrupt lysosomes in the cells, causing cell damage and release of toxic products into the joint. Joints and kidneys may be damaged by the sodium urate crystals. Excretion of excess urate is also inhibited by high lactate levels, for example the elevated lactate associated with the increase in NADH from metabolism of ethanol in alcoholism.
An acute attack of gout is treated with anti-inflammatory drugs, such as NSAIDS, glucocorticoids or colchicine, or drugs to inhibit renal reabsorption of urate. Allopurinol is ineffective in treatment of an acute attack but is used as a long-
term therapy for gout. Allopurinol is an irreversible inhibitor of xanthine oxidase, which catalyzes two final steps in the catabolism of purines to uric acid. Allopurinol treatment lowers uric acid synthesis while increasing excretion of earlier intermediates in the pathway. However, as allopurinol does not inhibit uric acid production completely, a mixture of hypoxanthine, xanthine and uric acid is excreted but in concentrations that are low enough to reduce the chance of sodium urate crystal formation.
Adenosine deaminase (ADA) deficiency is a rare autosomal recessive disorder. Adenosine deaminase catalyzes deamination of adenosine to inosine. Clinically ADA deficiency manifests as a severe combined immunodeficiency disorder (SCID). ADA deficiency was the subject of the first successful human somatic gene therapy trial reported in 1990. A retroviral vector was used to carry the normal ADA transgene intended to be expressed in lymphocytes. However, participants in the trial continued receiving exogenous ADA protein for ethical reasons, in case the gene therapy trial was unsuccessful.
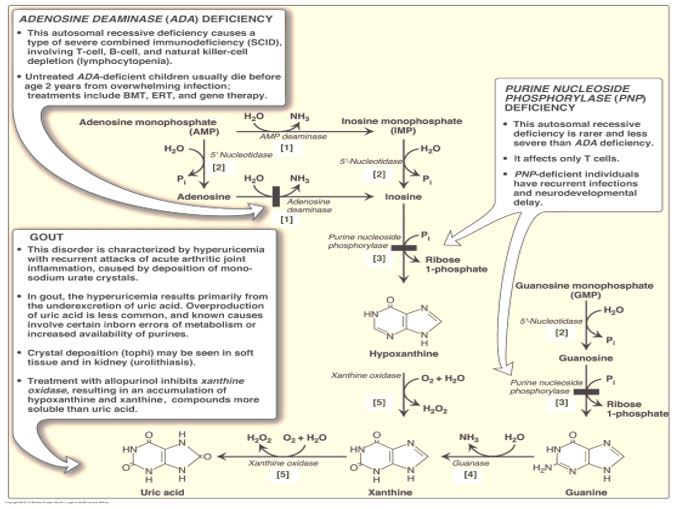
Figure 12.
A deficiency of ADA results in excess production of adenosine, causing a downstream effect of a 100-fold increase in dATP levels. Elevated dATP levels inhibits ribonucleotide reductase, leading to compromised production of deoxynucleotides and inhibition of proliferation of B and T lymphocytes in SCID. Immune dysfunction results from impairment of lymphocyte differentiation, function or viability. While this inhibition of ribonucleotide reductase in ADA deficiency contributes to inhibition of DNA synthesis and repair, the overall etiology of ADA deficiency-associated SCID involves many processes.
Purine nucleoside phosphorylase (PNP) deficiency, a rare autosomal recessive disorder, is characterized by severe to non-severe SCID. PNP deficiency, while less severe than ADA deficiency, is associated with neurological symptoms and increased risk of autoimmune disorders. PNP-catalyzed reactions utilize phosphate in removal of ribose from inosine or guanosine, generating ribose 1-P and either hypoxanthine or guanine, respectively.
Key points in purine and pyrimidine metabolism disorders and therapies
- Inhibition of thymidylate synthase with F-dUMP (from 5-FU) deprives the cell of a precursor for DNA, resulting in inhibition of DNA synthesis and repair.
- In Lesch Nyhan syndrome, HPRTase deficiency is associated with neurological dysfunction and excess de novo synthesis of purines causes increased uric acid and gout.
- Inhibition of xanthine oxidase with allopurinol reduces production of uric acid and is used in the long-term treatment of gout.
- Ribonucleotide reductase is required for making deoxyribonucleotides from ribonucleotides; inhibition of this enzyme will inhibit synthesis of new DNA.
- Adenosine deaminase (ADA) deficiency leads to excess dATP that inhibits ribonucleotide reductase. The severe combined immunodeficiency (SCID) that results in these patients is only partially explained by this mechanism.
Feedback:
