12 Amino Acid Derivatives
AMINO ACID DERIVATIVES
WWAMI FMR
Pamela Langer, PhD, WWAMI-WY
Session Learning Objectives:
SLO4: Describe the biochemical role of reduced and oxidized glutathione.
Amino Acids Derivatives, Overview
In addition to being precursors in protein synthesis, gluconeogenesis, and lipid synthesis, amino acids play important metabolic roles as precursors for other amino acids, nucleotides, and various biological mediators. The physiological and clinical significance of these amino acid derivatives will be discussed briefly in this chapter along with their biosynthesis. For example, creatine is synthesized from two amino acids and the methyl group from S-adenosylmethionine (SAM). Creatine kinase catalyzes the conversion of creatine to creatine phosphate, an energy storage molecule, that spontaneously converts to creatinine, an analyte in assessing kidney function.
The 20 proteinogenic amino acids are organized into groups according to properties of their side chains (R groups). Identifying an amino acid with a group highlights some of its functional properties as well as potential consequences when substituted in a protein because of a genetic change. For example, substitution of an amino acid containing a positively-charged side chain with an amino acid with a negatively-charged side chain may alter protein structure. Substitution of a cysteine with another amino acid may cause the absence of appropriate disulfide bonding critical to formation of a functional protein.
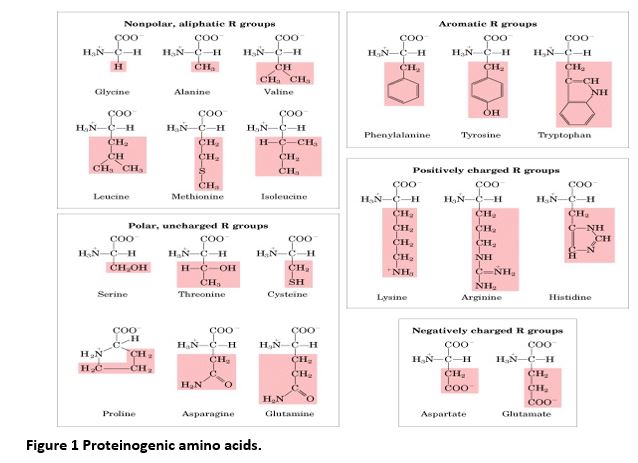
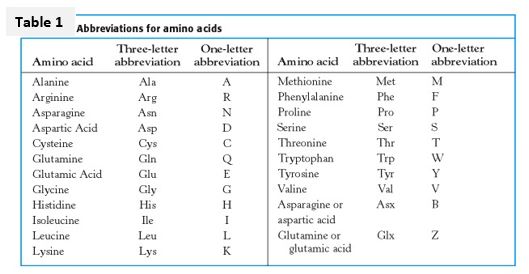
Knowing the full name, 3-letter, 1-letter, and group designations for the 20 standard amino acids will facilitate recognition of types of genetic changes and comprehension of certain topics in research seminars and papers in medical biochemistry and genetics.
Coenzymes play a significant role in the synthesis of amino acids and their derivatives. Multiple coenzymes are required in some pathways, for example, the synthesis of catecholamines from tyrosine requires tetrahydrobiopterin (BH4), pyridoxal phosphate (PLP, a derivative of vitamin B6), ascorbate (vitamin C) and the co-substrate S-adenosylmethionine (SAM) as the methyl group donor in the final methylation step from norepinephrine to epinephrine. Furthermore, a single coenzyme may be required by multiple pathways. A deficiency in the coenzyme can result in a disorder related to one caused by deficiency of the enzyme using that coenzyme. For example, tetrahydrobiopterin (BH4) deficiency causes a reduction of activity in the BH4-dependent phenylalanine hydroxylase reaction and results in a form of phenylketonuria (PKU). Disorders related to defects in the synthesis of amino acid derivatives will be discussed in more detail in another session.
Amino acid derivatives considered in the first two learning objectives for this session are each derived from single amino acids, with tyrosine-derived derivatives considered separately, followed by those derivatives requiring more than one amino acid for their synthesis. The molecules introduced here will be revisited in other parts of the foundational courses.
SLO 1: Describe the biosynthetic origin and basic function of the biological mediators histamine, gamma-aminobutyric acid (GABA), serotonin and nitric oxide.
Biosynthetic pathways for histamine, gamma-aminobutyric acid (GABA), serotonin, and the catecholamines involve decarboxylation reactions that employ the coenzyme pyridoxal phosphate (PLP), a derivative of vitamin B6. Note that PLP is also used in many other types of metabolic reactions such as aminotransferases and biosynthesis of cysteine or the heme ring.
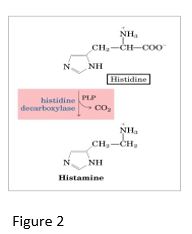
Histamine:
Histamine, derived from histidine, mediates multiple physiological processes via binding to different histamine receptors that are G-protein coupled receptors (GPCRs), H1-H4 in humans. For example, when hist
amine is released during an allergic response, binding to the H1 receptor results in cell signaling that stimulates mucus secretion, increases vasodilation and vascular permeability and can lead to bronchoconstriction in allergy-induced asthma. In contrast, when histamine is released during the digestive process, binding to the H2 receptor stimulates a different GPCR-mediated cell signaling pathway that results in release of HCl into the gastric lumen. Histamine H2 receptor antagonists such as ranitidine are used clinically to decrease acid production in the stomach.
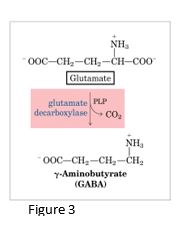
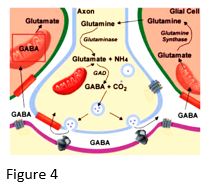
Gamma-aminobutyric acid (GABA)
GABA, an inhibitory neurotransmitter in the central nervous system, is synthesized from the amino acid glutamate (one of the main excitatory neurotransmitters) using pyridoxal phosphate, a derivative of Vit B6. Underproduction of GABA is associated with epileptic seizures, and GABA analogs (e.g. barbiturates) are used in the treatment of epilepsy.
In addition, Gabapentin, a GABA analog, is used in treatment of neuropathic pain with diabetic neuropathy. GABA levels can also be raised by giving inhibitors of the enzyme that degrades GABA (GABA aminotransferase), resulting in an increase in activity of inhibitory neurons.
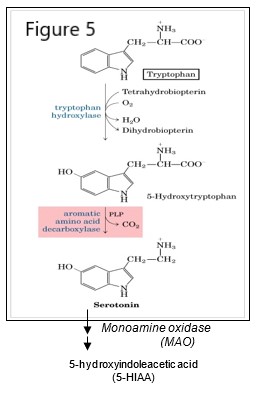
Serotonin
Tryptophan is a precursor in the synthesis of serotonin, a monoamine neurotransmitter involved in mood regulation.
Serotonin is in turn a precursor for melatonin, a hormone involved in sleep regulation and synthesized mainly in the pineal gland. Tryptophan is also used in the synthesis of niacin (nicotinic acid, a form of vitamin B3), and therefore the precursor for synthesis of nicotinamide adenine dinucleotide (NAD+) and associated forms NADH, NADP+ and NADPH. A chronic, severe deficiency of tryptophan or niacin can lead to pellagra, a disease affecting the skin, digestive and nervous systems.
Serotonin is synthesized in a two-step pathway that utilizes the coenzyme tetrahydrobiopterin (BH4) in and pyridoxal phosphate (PLP). Serotonin is degraded to 5-hydroxyindolacetic acid (5-HIAA) via the action of monoamine oxidase (MAO), an enzyme also involved in degradation of catecholamine neurotransmitters.
Serotonin levels may be assessed by measuring the level of 5-HIAA, for example for a case where there is a suspected serotonin-secreting tumor.
Inhibition of serotonin reuptake into a presynaptic neuron may be used to increase the level of serotonin in the synapse in the treatment of depression.
Although the names are somewhat similar, melatonin is derived from tryptophan and melanin is a pigment derived from tyrosine. Melatonin and melanin have very different functions.
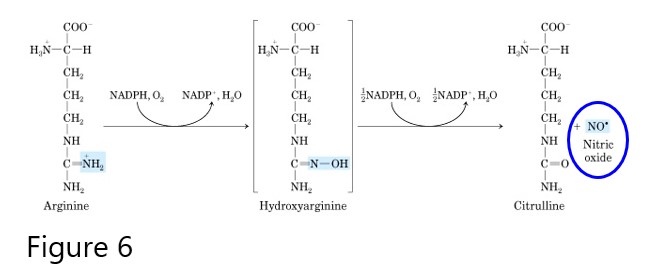
Nitric oxide
Nitric oxide (NO) is a short-range, short-lived signaling molecule. NO is synthesized from arginine via a nitric oxide synthase (NOS)-dependent pathway yielding citrulline and nitric oxide. A NOS-independent pathway, involving nitrate (NO3-) and nitrite (NO2-), may also generate nitric oxide from food rich in nitrates, such as leafy green vegetables and beets. Many different physiological responses are mediated by different NOS isozymes such as eNOS, a vascular endothelial isoform, nNOS, a neuronal isoform, or iNOS, an inducible isoform that plays a role in immune defense mechanisms.
Nitric oxide diffuses readily through membranes, however its high reactivity limits diffusion to about 1 mm from the site of synthesis.
Soluble NO binds the heme iron of guanylyl cyclase that catalyzes conversion of GTP to cyclic GMP (cGMP), a powerful biological mediator in signaling pathways. NO has several effects, but it may be considered as a smooth muscle relaxant that promotes dilation of blood vessels. The mechanism by which cGMP promotes vasodilation may involve reduction in cytosolic Ca2+, resulting in less Ca2+ available to promote smooth muscle contraction. Drugs such as nitroglycerin have been used to relieve angina, as the slow release of NO upon degradation of nitroglycerin causes increased cGMP, resulting in reduced myocardial wall tension, dilation of epicardial coronary vessels, and increased blood flow in collateral vessels.
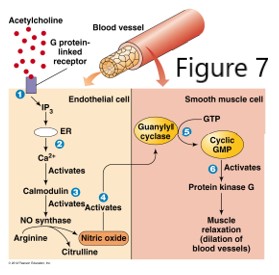
Cyclic GMP is degraded by phosphodiesterases, so if the goal is to increase cGMP, phosphodiesterase (PDE) inhibitors can be used. For example, cGMP levels are increased by inhibiting the breakdown of cGMP with the drug sildenafil, a PDE5 inhibitor that enhances the effect of NO, resulting in penile smooth muscle relaxation and increased blood flow to erectile tissue. Details of the effect of NO on smooth muscle and the heart will be discussed elsewhere in the curriculum.
SLO 2: Describe the biosynthetic origin and basic function of molecules derived from tyrosine: thyroid hormone, melanin, and the catecholamines dopamine, norepinephrine, and epinephrine.
Thyroid hormone
Thyroid hormone (TH) plays an important role in growth and metabolism. TH refers collectively to two forms: T4 is tetraiodinated thyronine, a prehormone that is deiodinated to the active form T3 (triiodothyronine). Note that calcitonin, made in the thyroid parafollicular cells (C-cells) is involved in calcium and phosphate homeostasis but is not included in the designation “thyroid hormone.”
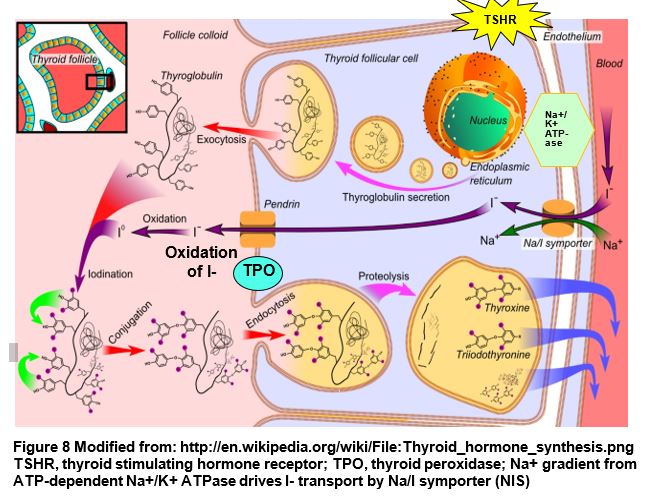
Over- or underproduction of thyroid hormone (hyper- or hypothyroidism) will be discussed in detail in other foundation blocks, however it is important to connect the biochemistry and physiology with clinicalapplication. For example, in treatment of hyperthyroidism, thyroid hormone synthesis may be reduced by inhibiting thyroid peroxidase (TPO) with methimazole or propylthiouracil (PTU). Because the majority of serum iodide is concentrated by the thyroid, thyroid cells may be selectively destroyed by administering the 131I isotope that will be concentrated in the thyroid.
Thyroid hormone is synthesized by the thyroid in response to hormonal regulation and availability of iodide. Thyroid hormone biosynthesis involves the following steps.
- Serum iodide (I-) enters the follicular cell via a Na/I symporter against a concentration gradient, using the Na/K ATPase to generate the sodium gradient to drive “iodide trapping.”
- Thyroglobulin (TG) is exocytosed, and iodide is transported via the apical membrane anion transporter Pendrin, to the amorphous acellular colloid portion of the thyroid.
- Thyroid peroxidase (TPO) oxidizes I- to Io, used to iodinate tyrosines within thyroglobulin.
- The side chain of either a monoiodinated (MIT) or diiodinated (DIT) tyrosine is conjugated with a diiodinated tyrosine in thyroglobulin to form triiodothyronine (T3) or tetraiodothyronine (thyroxine) (T4) residues in thyroglobulin.
- In response to signals, colloid containing iodinated thyroglobulin is endocytosed by the follicular cell, and proteolysis of thyroglobulin releases MIT, DIT, T3 and T4.
- T3 and T4 are secreted from the cell and are bound to serum carrier proteins.
- T4 (pre-hormone) is converted peripherally to T3 (active) by iodothyronine (T4) deiodinase, a selenoprotein, or to inactive reverse T3 (rT3), depending on physiological conditions.
- Conversion to rT3 reduces the reservoir of T4 that can be converted to T3.
- In the serum, thyroid hormone is transported by thyroxine binding globulin (TBG), transthyretin (TTR) (previously called thyroxine binding pre-albumin) and albumin. Thyroid hormones must be free of carrier proteins (unbound) to be active (e.g. free T3).
Melanin
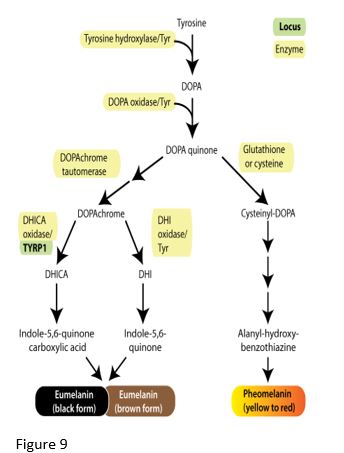
Various forms of melanin are synthesized from tyrosine, with the enzyme tyrosinase catalyzing the first step. Skin color results from pigment (melanin granules) in keratinocytes and melanocytes in superficial skin layers. Relative proportions of melanin variants determine the color of skin and hair. Dark hair contains predominantly eumelanin while blond hair results from a small amount of eumelanin production. Red hair results from production of pheomelanin with very little eumelanin production. Binding of melanocyte stimulating hormone (MSH) to its receptor (melanocortin 1 receptor or MC1R) promotes eumelanin production by activating enzymes in the eumelanin biosynthetic pathway. However, MC1R variants do not bind MSH well, and consequently the cell makes pheomelanin and produces only a small amount of eumelanin. People with red hair may be homozygous for a MC1R allelic variant, however many other genes affect the amount of expression of the enzymes involved in eumelanin and pheomelanin production.
Catecholamines
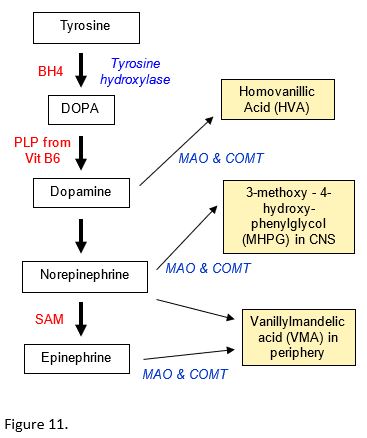
Three monoamine neurotransmitters are synthesized from tyrosine in the catecholamine pathway: dopamine, norepinephrine (NE, noradrenaline) and epinephrine (Epi, adrenaline). The pathway involves the coenzymes Tetrahydrobiopterin (BH4); Pyridoxal phosphate (PLP), derived from Vit B6; Ascorbate (Vitamin C, used in dopamine to NE reaction); and S-adenosylmethionine (SAM) (methyl group donor in NE to E reaction).
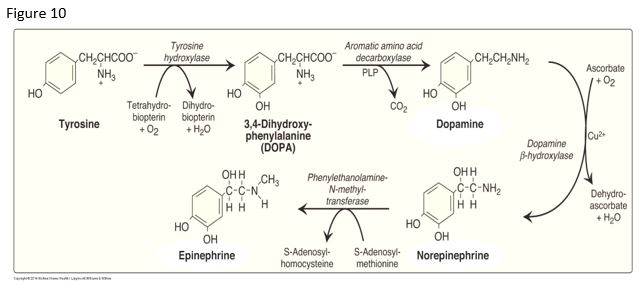
Monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) are involved in the degradation of dopamine, norepinephrine (NE), and epinephrine (E).
The catecholamine degradation products may be assessed to determine neurotransmitter levels. For example, degradation of NE results in 3-methoxy-4-hydroxy-phenylglycol (MHPG) in the CNS. Monoamine oxidase inhibitor drugs (MAOIs) may be used in the treatment of certain depressive disorders.
Patients with Parkinson disease may exhibit a dopamine deficiency, providing the basis for treating these patients with the drug L-DOPA, which crosses the blood brain barrier.
SLO 3: Discuss the biosynthetic relationship between creatine and creatine phosphate and the use of creatinine as a clinical analyte.
Creatine, creatine kinase, creatinine phosphate, creatinine
Three different amino acids are used to synthesize creatine. Phosphorylation of creatine by creatine kinase produces creatine phosphate (phosphocreatine), an important reservoir for a high energy phosphate bond and used in the conversion of ADP to ATP.
Creatine kinase (CK) is an important enzyme for regeneration of ATP. Serum levels of various CK isoforms have been used diagnostically during suspected acute myocardial infarction (AMI). However, assessment of changing levels of total CK and CK isozymes in an AMI has been largely replaced with the use of cardiac-specific forms of troponin subunits (cTnI or cTnT).
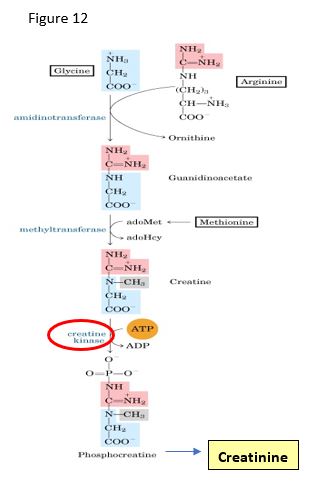
Creatinine is synthesized via a spontaneous, nonenzymatic dehydration of creatine phosphate, mostly in skeletal muscle. Creatinine is an important clinical analyte for two main reasons: (i) creatinine levels are a function of muscle mass and show little response to dietary change; (ii) creatinine is freely filtered by the kidney (i.e. not reabsorbed from the renal filtrate). Serum creatinine is used as first test for the capacity of the kidney to filter substances from the blood (glomerular filtration) and is used before surgical procedures to assess the patient’s ability to clear the anesthetics. A creatinine clearance test is a more detailed measure of renal filtering capability, namely the glomerular filtration rate (GFR); however, this test is rarely done currently.
SLO 4: Describe the biochemical role of reduced and oxidized glutathione.
Glutathione (GSH) acts as a redox buffer, serving as a reducing agent, playing a role in toxin removal, and protecting against oxidative stress. Superoxide radical and hydrogen peroxide are generated when the cell is exposed to stressors such as certain drugs, radiation or normal mitochondrial respiration.
Glutathione synthesis
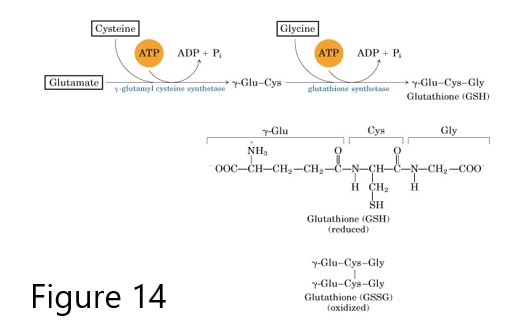
Glutathione, is synthesized from 3 amino acids (Glu, Cys, Gly), and its reduced form GSH protects cells from oxidative damage during normal cell processes and contributes to the processing of certain xenobiotics including toxins and drugs. In an energy-requiring process, the glutamate of the tripeptide is attached via the gamma-carbon of its R group. Consequently, glutathione is also designated as Gamma-Glu-Cys-Gly. Glutathione cycles between the reduced thiol form (GSH) and the oxidized, disulfide bonded form (GSSG), also called glutathione disulfide. The enzyme glutathione peroxidase catalyzes conversion of hydrogen peroxide (H2O2) and GSH to water and GSSG.
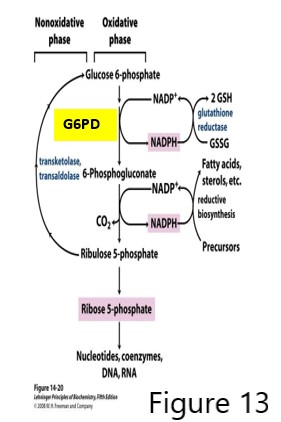
GSH regeneration is catalyzed by glutathione reductase using GSSG and NADPH generated by the pentose phosphate pathway (PPP, also called hexose monophosphate shunt, which also produces pentose phosphate sugars used in nucleotide synthesis.)
The first reaction of the PPP, catalyzed by glucose 6-phosphate dehydrogenase (G6PD), is critical for producing an adequate amount of NADPH used in reductive biosynthetic pathways and importantly in the generation of GSH. Deficiency in GSH regeneration may result in elevated reactive oxygen species (ROS) and oxidative damage. Individuals deficient in G6PD and exposed to high oxidative stress, may present with serious clinical issues because of the deficiency of NADPH and consequently, deficiency of GSH.
Decreased glutathione in G6PD deficiency
As the only source of NADPH in the RBC is the pentose phosphate pathway, a G6PD defect causes decreased NADPH and therefore low GSH production in the RBC. RBCs carry high levels of O2 and are susceptible to oxidative stress. Furthermore, RBCs are unable to regenerate enzymes because they have no nucleus. Although some people with a G6PD deficiency never exhibit symptoms, G6PD deficiency symptoms are worsened with high oxidative stress caused by taking certain drugs (e.g. the anti-malarial primaquine), having certain infections, eating fava beans, or being exposed to other environmental conditions that increase oxidative stress. In situations of high oxidative stress with low GSH, the most common manifestation of G6PD deficiency is hemolytic anemia.
X-linked recessive G6PD deficiency is the most common genetic deficiency, with ~400 million cases worldwide and highest prevalence in people of African, Asian and Mediterranean descent. In the US, 1 in 10 males of African American descent have a G6PD deficiency, and females are usually affected only if they have 2 defective G6PD alleles.
G6PD deficiency confers partial protection against malaria, a reason promulgated to explain the high prevalence of G6PD defects in the world. There are over 300 variants of G6PD alleles, resulting in a polymorphic clinical presentation, from mild to severe. Screening for G6PD deficiency is not typically part of newborn screening panels.

Glutathione in toxin removal
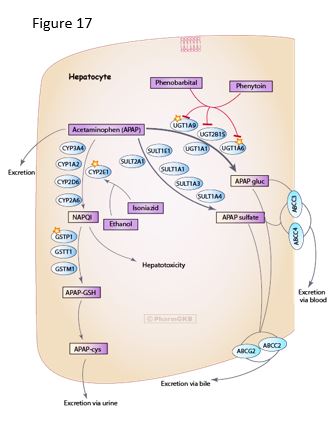
GSH is essential in normal metabolism as well as for the processing of toxins. For example, acetaminophen is processed via multiple pathways, including glucuronidation or sulfation, or to a lesser extent, via oxidation by P450 enzymes, generating a toxic catabolite NAPQI that is detoxified by conjugation with GSH, spontaneously or catalyzed by glutathione S-transferase (GST).
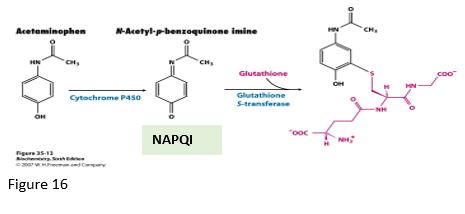
In cases of overdose, high levels of acetaminophen can cause hepatocellular necrosis and liver damage from alteration of the cellular redox potential and damage from the NAPQI toxin, especially if the GSH levels are decreased in starvation. However, toxicity may be exacerbated if the level of activity of certain cytochrome P450 (CYP) enzymes is increased. For example, ethanol will increase the activity of and stabilize CYP2E1, causing increased activity in the pathway from acetaminophen to NAPQI. Furthermore, genetic polymorphism in CYP2E1 allelic variants can cause individual variation in drug metabolism and susceptibility to liver damage. Consequently, it would be difficult to predict which individuals might have a serious adverse drug reaction if ingesting ethanol and acetaminophen.
Feedback:
