10 Introduction to the Hypothalamus
Introduction to the Hypothalamus
The hypothalamus is the primary regulator of preganglionic ANS neurons of the brain stem and spinal cord. It innervates the nucleus of the solitary tract in the medulla, which integrates sensory input from internal organs to coordinate autonomic output. Among its numerous and diverse roles, the hypothalamus serves as the master control center for most endocrine systems.
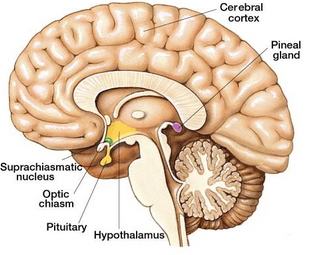
The hypothalamus sits adjacent to the dorsal thalamus bordering the walls of the third ventricle. Despite being a relatively small structure, the hypothalamus is the primary bridge between the central nervous system and the endocrine system, providing a mechanism through which emotions can affect peripheral systems, and vice versa. The hypothalamus is capable of integrating both neural and endocrine signals and can provide neural output to the CNS and ANS, as well as both direct and indirect (i.e., via secretory organ innervation) endocrine outputs.
 Deviations beyond a normal range in almost any homeostatic parameters are detected by specialized sensory neurons of the hypothalamus. However, chemoceptors for a given substance in the hypothalamus are often distinct from visceral receptors for the same substance. The hypothalamus can generate an appropriate response through direct and indirect regulation of numerous systems.
Deviations beyond a normal range in almost any homeostatic parameters are detected by specialized sensory neurons of the hypothalamus. However, chemoceptors for a given substance in the hypothalamus are often distinct from visceral receptors for the same substance. The hypothalamus can generate an appropriate response through direct and indirect regulation of numerous systems.
The hypothalamus can react to deviations with a humoral response by directly releasing hormones into open circulation, or by altering the secretion by other glands. The hypothalamus can also adjust the balance between SNS and PSNS activity as part of a visceromotor response. Lastly, the hypothalamus can induce changes in motivated behavior that contribute to the restoration of homeostasis, a somatic motor response.
The majority of endocrine regulation by the hypothalamus is achieved through hypothalamic control of the pituitary gland. The pituitary gland is a small structure cradled by bone in the base of the skull. It is physically tethered to the hypothalamus by stalk called the infundibulum, which stems from the hypothalamus at the median eminence. The pituitary is divided into two lobes, anterior and posterior, secretion from each of which is regulated by the hypothalamus in different ways.
Hypothalamic neurons secrete two types of 9-amino acid peptide hormones (oxytocin and vasopressin) directly into circulation at the posterior pituitary. Both of these neuropeptides are released by magnocellular neurosecretory cells of the hypothalamus, which project down the infundibulum to capillary beds in the posterior pituitary (also called the neurohypophysis). Vasopressin (VP), also called antidiuretic hormone (ADH), has numerous effects, both central and peripheral; one of its primary peripheral effects is to increase water retention by the kidneys. Among its numerous effects within the nervous system, oxytocin (OT) stimulates milk ejection by the mammary glands and uterine contractions during childbirth. Within the nervous system, oxytocin and vasopressin are involved in bonding behaviors (remember that hormones are often used as neurotransmitters). Oxytocin has received considerable attention because of its implication in mother-child bonding, but this role of oxytocin does not generalize between sexes. In fact, while oxytocin is implicated in bonding behavior in females, it is vasopressin that is implicated in bonding behavior in males. This is a distinct hypothalamic inter-sex difference.
In addition to these hormones secreted into open circulation, parvocellular neurosecretory cells of the hypothalamus also secrete regulatory hormones into a unique portal system connecting a hypothalamic capillary bed to a capillary bed in the anterior pituitary (also called the adenohypophysis). Hypothalamic secretion into this hypophyseal portal system can enhance or inhibit the anterior pituitary’s secretion of tropic hormones into open circulation.
The most basic division of the hypothalamus is into lateral and medial regions. The lateral hypothalamus (LH) contains mostly fibers, whereas the medial hypothalamus contain most of the hypothalamic nuclei. The medial nuclei closest to and bordering the third ventricle receive most of their neural input from the lateral area, and the rest of the medial area, but they also receive input from other brain regions ranging from the brainstem to cortex. These nuclei have both endocrine outputs via neurosecretory cells as well as neural projections that regulate ANS outputs to visceral organs. The remaining nuclei of the medial area, and the neurons of the lateral area, form extensive connections with the rest of the CNS, endowing their ability to participate in various behavioral processes that promote homeostasis. As such, the hypothalamus as a whole contributes to homeostasis within the body both by altering internal processes and by driving behaviors that contribute to internal homeostasis (e.g., seeking shade on a hot day, or seeking a meal when hungry).
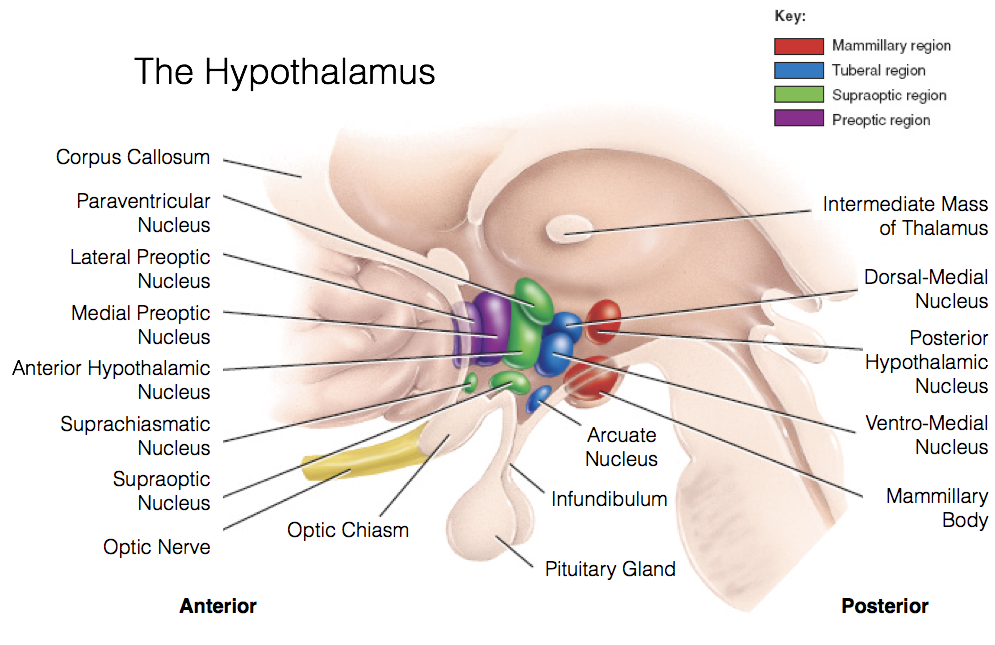
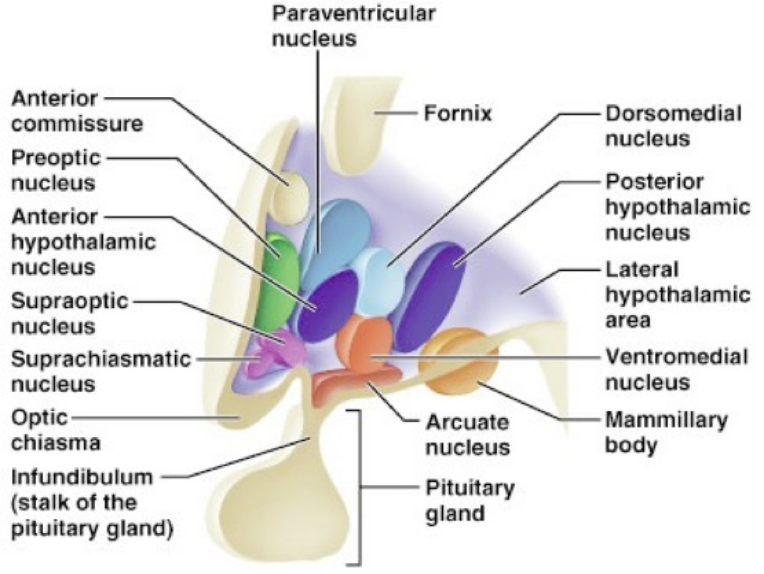 The medial hypothalamus is subdivided into four areas, the pre-optic area, supra-optic area, tuberal area, and mammillary region. Each of these medial hypothalamic areas contains multiple nuclei.
The medial hypothalamus is subdivided into four areas, the pre-optic area, supra-optic area, tuberal area, and mammillary region. Each of these medial hypothalamic areas contains multiple nuclei.
The preoptic area contains two nuclei largely involved in fluid balance. The lateral preoptic nucleus monitors salt, nitrogen, and glucose levels using osmoreceptors. The ventral region of this nucleus, the ventrolateral preoptic nucleus, plays a large role in regulating sleep and wakefulness. The medial preoptic nucleus has osmoreceptors as well. Furthermore, due to the fact that the medial pre-optic area is larger in male rats compared to females, it is often called the sexually dimorphic nucleus of the preoptic area (SDN-POA). As such, the medial preoptic nucleus appears to be involved in the predominantly masculine aspects of sexual behavior.
The supraoptic area is composed of the anterior hypothalamus, suprachiasmatic nucleus, supraoptic nucleus, paraventricular nucleus, and the organum vasculorum lamina terminalis. The anterior hypothalamus is divided into three interstitial nuclei, the third of which (interstitial nucleus of the anterior hypothalamus-3; INAH-3) is also sexually dimorphic, with (limited) evidence indicating that size is related to sexual preference. The suprachiasmatic nucleus (SCN) is innervated by intrinsically photosensitive retinal ganglion cells. This enables the SCN to change its functioning in response to light exposure to align various biological rhythms to light-dark cycles. The supraoptic nucleus (SON) and paraventricular nucleus (PVN) are somewhat interchangeable and serve very similar roles. Both contain magnocellular neurosecretory cells that release oxytocin and vasopressin through the posterior pituitary. Both also contain parvocellular neurosecretory cells that secrete tropic-hormone-releasing hormones to the anterior pituitary. Lastly, the organum vasculorum lamina terminalis (OVLT) sits at the rostral base of the third ventricle, and plays a role in maintaining fluid balance through use of osmoreceptors. The OVLT also lacks a blood-brain-barrier, enabling detection of hormonal signals that may otherwise fail to reach the CNS.
The tuberal area sits superior to the pituitary at the medial eminence, and contains the ventromedial hypothalamus, dorsomedial hypothalamus, arcuate nucleus, and periventricular nucleus. The periventricular nuclei immediately surround the third ventricle, and produce numerous hypothalamic hormones (e.g., TRH, GH, GnRH, etc.). They also lack a blood-brain-barrier. The ventromedial hypothalamus (VMH) is largely involved in the regulation of feeding behavior, and also exhibits sexual dimorphism, being larger in female rats compared to male rats. The VMH has been generally regarded as a satiety center in the brain, because lesions cause overeating of particular food types (specifically carbohydrates), which leads to obesity. The dorsomedial hypothalamus (DMH) participates primarily in the same processes as the VMH. Lastly, the arcuate nucleus is also largely involved in feeding behavior by regulating the production of both signals promoting and inhibiting feeding behavior.
The mammillary region generally refers to the mammillary bodies themselves and the posterior nucleus. Both the mammillary bodies and posterior nucleus are involved in thermoregulation and autonomic functioning. Notably, they are also linked to the hippocampus via the fornix, and appear to serve a role in memory consolidation.
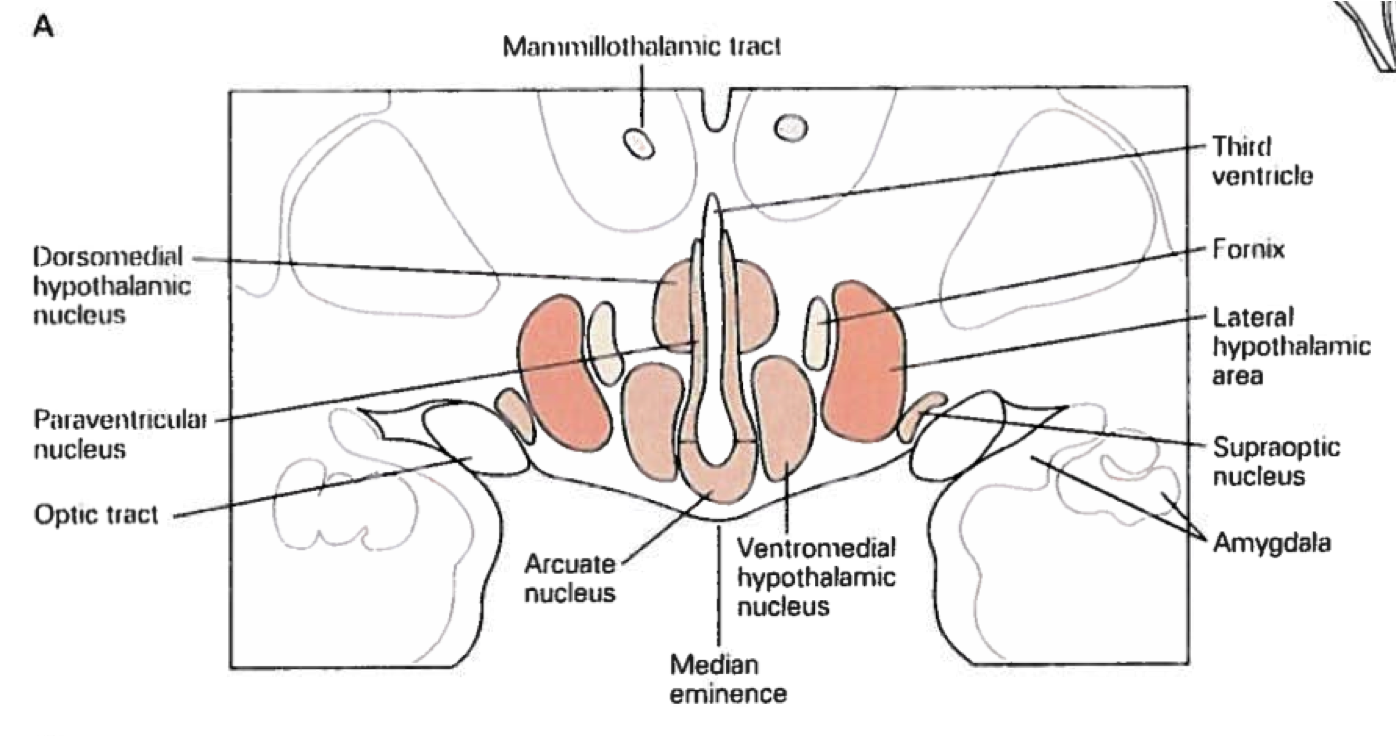
In contrast to the medial hypothalamus, the lateral hypothalamus is not organized into well-defined nuclei. However, fibers passing through the LH, as well as the neurons within the LH, play a large role in motivated behaviors, particularly those involved in feeding behavior. Whereas the VMH lesions precipitate obesity, LH lesions lead to anorexia. Furthermore, in animal models, stimulation of the LH will induce eating following a degree of consumption that would otherwise lead to complete satiation.
Despite its small size, the complexity of information processing achieved by the hypothalamus is staggering. Its role in preserving homeostasis is of vital importance, especially with regards to the maintenance of energy, fluid, and temperature balances, and its establishment of biorhythms.

Feedback/Errata