5 Endocrine Regulation of Reproductive Systems
Endocrine Regulation of Reproductive Systems
In the last unit, we focused primarily on homeostatic hormones responsible for maintaining a stable environment. However, we transitioned to episodic hormones when discussing hormones involved in digestion. In this unit, we will continue our discussion of episodic, non-homeostatic hormones, and begin to examine the neuroendocrine roles of the hypothalamus in more depth.
HPT Axis
One of the primary homeostatic systems discussed in the first unit was the Hypothalamus-pituitary-thyroid axis (HPT axis). Thyrotropin releasing hormone (TRH) is secreted by the hypothalamus, which stimulates the release of thyroid-stimulating hormone (thyrotropin; TSH) by the anterior pituitary, which stimulates the production and release of triiodothyronine (T3) and thyroxin (T4) by the thyroid. T3 then exerts feedback inhibition upon both the hypothalamic release of TRH and pituitary release or TSH. T4, on the other hand, only exerts feedback upon the anterior pituitary, not the hypothalamus.
HPA Axis
In the hypothalamic-pituitary-adrenal gland axis (HPA-axis), the paraventricular nucleus of the hypothalamus secretes the peptide hormone corticotropin-releasing hormone (CRH). CRH is also synthesized by T-cells of the immune system, as well as the placenta during pregnancy. In addition to homeostatic release of CRH, CRH secretion increases in response to stress, where stress is generally defined as any condition that seriously perturbs physiological (or by extension psychological) homeostasis. CRH synthesized by the hypothalamus is released into capillaries that converge into the hypophyseal portal system, a portal through the infundibulum connecting hypothalamic capillaries to the anterior pituitary (i.e., the adenohypophysis). CRH in this closed-circulation stimulates the release of adrenocorticotropic hormone (ACTH) by the anterior pituitary. ACTH stimulates the production of corticosteroids (i.e., steroid hormones produced in the adrenal cortex). One class of these corticosteroids, glucocorticoids, exert negative feedback upon the release of ACTH by the anterior pituitary and upon the release of CRH by the hypothalamus. We will return to the effects of glucocorticoids later.
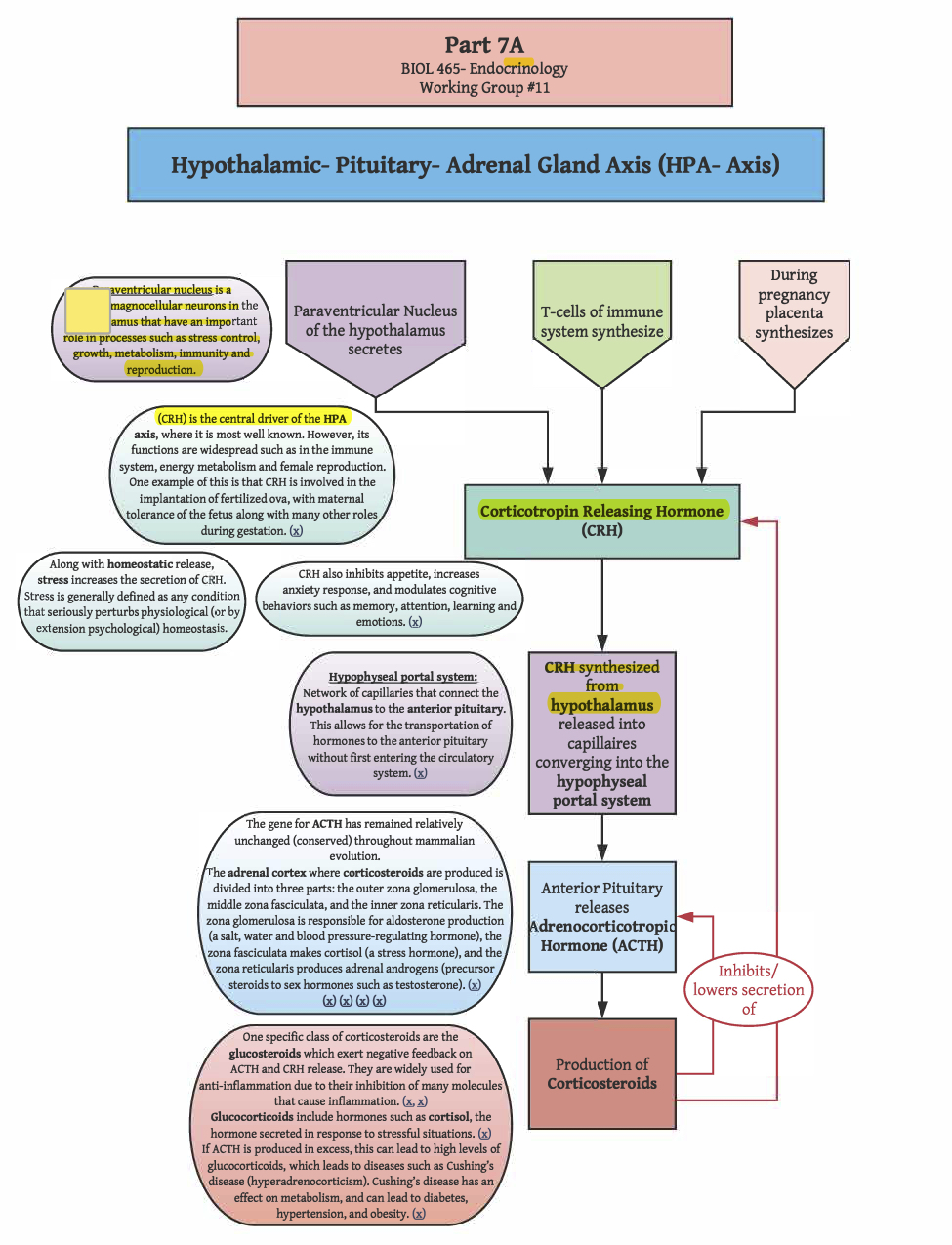
 ACTH is a peptide hormone cleaved from proopiomelanocortin (POMC). POMC is a 241 amino acid peptide synthesized in the anterior pituitary (though it is also transcribed in other regions, such as the arcuate nucleus of the hypothalamus, the brain stem, and in melanocytes in the skin). Peptides cleaved from POMC, called melanocortins, are packaged into dense core vesicles for release through exocytosis. In addition to ACTH, sections of POMC can be cleaved to yield other peptide hormones, such as the melanocyte-stimulating hormones (MSH; also called melanotropins), of which there are three types (αMSH, βMSH, γMSH). αMSH, βMSH, and γMSH are all cleaved from different regions of POMC (i.e., all three variants of MSH can be produced from a single POMC). Other regions of POMC are cleaved to yield other melanocortins, including β-lipotropin, γ-lipotropin, and β-endorphin. While MSHs and ACTH stimulate many of the same melanocortin receptors (MCRs), only ACTH stimulates the melanocortin type 2 receptors (MC2Rs; Agouti-related protein and agouti-signaling protein are antagonist peptides at MC2R). MC2R is primarily expressed in the zona fasciculata of the adrenal cortex, the layer of the adrenal cortex responsible for glucocorticoid synthesis. MC2R is also expressed to a lesser degree in the skin. Activation of MC2Rs on the adrenal gland stimulates glucocorticoid synthesis, and also has a minor impact on mineral corticoid synthesis through stimulation of precursor production (i.e., MC2R activation on cells of the zona glomerulosa promotes the production of deoxycorticosterone, which is a precursor to corticosterone, which can be converted to aldosterone). There are very few ACTH receptors on the zona glomerulosa, and they are quickly saturated, causing ACTH-driven aldosterone synthesis to rapidly reach its peak effects. Thus, ACTH effects on circulating levels of aldosterone are minimal, especially in contrast to the proportional effects of ACTH on circulating levels of glucocorticoids. For ACTH to bind to an MC2R, a melanocortin-2 receptor accessory protein-1 (MRAP1) must also be bound. Without MRAP1, the receptor will never be translocated to the surface of the cell membrane. Rather, MC2R will be degraded in the ER. Thus, by regulating the availability of MRAP1, the effects of ACTH can be regulated. In fact, cyclic changes in MRAP1 expression may contribute to the diurnal cortisol rhythm.
ACTH is a peptide hormone cleaved from proopiomelanocortin (POMC). POMC is a 241 amino acid peptide synthesized in the anterior pituitary (though it is also transcribed in other regions, such as the arcuate nucleus of the hypothalamus, the brain stem, and in melanocytes in the skin). Peptides cleaved from POMC, called melanocortins, are packaged into dense core vesicles for release through exocytosis. In addition to ACTH, sections of POMC can be cleaved to yield other peptide hormones, such as the melanocyte-stimulating hormones (MSH; also called melanotropins), of which there are three types (αMSH, βMSH, γMSH). αMSH, βMSH, and γMSH are all cleaved from different regions of POMC (i.e., all three variants of MSH can be produced from a single POMC). Other regions of POMC are cleaved to yield other melanocortins, including β-lipotropin, γ-lipotropin, and β-endorphin. While MSHs and ACTH stimulate many of the same melanocortin receptors (MCRs), only ACTH stimulates the melanocortin type 2 receptors (MC2Rs; Agouti-related protein and agouti-signaling protein are antagonist peptides at MC2R). MC2R is primarily expressed in the zona fasciculata of the adrenal cortex, the layer of the adrenal cortex responsible for glucocorticoid synthesis. MC2R is also expressed to a lesser degree in the skin. Activation of MC2Rs on the adrenal gland stimulates glucocorticoid synthesis, and also has a minor impact on mineral corticoid synthesis through stimulation of precursor production (i.e., MC2R activation on cells of the zona glomerulosa promotes the production of deoxycorticosterone, which is a precursor to corticosterone, which can be converted to aldosterone). There are very few ACTH receptors on the zona glomerulosa, and they are quickly saturated, causing ACTH-driven aldosterone synthesis to rapidly reach its peak effects. Thus, ACTH effects on circulating levels of aldosterone are minimal, especially in contrast to the proportional effects of ACTH on circulating levels of glucocorticoids. For ACTH to bind to an MC2R, a melanocortin-2 receptor accessory protein-1 (MRAP1) must also be bound. Without MRAP1, the receptor will never be translocated to the surface of the cell membrane. Rather, MC2R will be degraded in the ER. Thus, by regulating the availability of MRAP1, the effects of ACTH can be regulated. In fact, cyclic changes in MRAP1 expression may contribute to the diurnal cortisol rhythm.
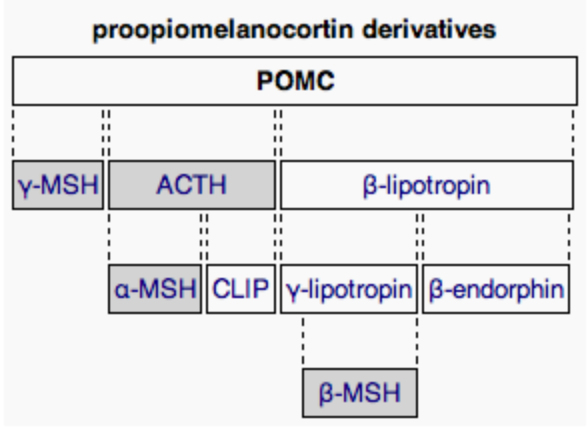
 The development of the adrenal gland sheds light onto its distinct divisions, which give rise to different types of corticosteroids. Development begins with the formation of the adrenogonadal primordium (AGP), a shared developmental origin for both the adrenals and gonads that are crucial in maintaining homeostasis of mammals. AGP will then divide into the fetal gonad and fetal adrenal tissue, leading to different pathways of further development. In fetal adrenal tissue, the adrenal medulla will form in the center from the neural crest cells migrated into the adrenal tissue. In other words, the adrenal medulla is directly innervated, or supplied by sympathetic nerves, which drive the release of catecholamines that initiate fight-or-flight responses (epinephrine, norepinephrine, and dopamine). and other crucial hormonal responses. The fetal adrenal tissue then develops into three distinct secretory layers, each with different cell types. Surrounding the adrenal medulla is the zona reticularis, responsible for the synthesis of adrenal androgens including dehydroepiandrosterone (DHEA) and hormone precursors to testosterone and estrogens.
The development of the adrenal gland sheds light onto its distinct divisions, which give rise to different types of corticosteroids. Development begins with the formation of the adrenogonadal primordium (AGP), a shared developmental origin for both the adrenals and gonads that are crucial in maintaining homeostasis of mammals. AGP will then divide into the fetal gonad and fetal adrenal tissue, leading to different pathways of further development. In fetal adrenal tissue, the adrenal medulla will form in the center from the neural crest cells migrated into the adrenal tissue. In other words, the adrenal medulla is directly innervated, or supplied by sympathetic nerves, which drive the release of catecholamines that initiate fight-or-flight responses (epinephrine, norepinephrine, and dopamine). and other crucial hormonal responses. The fetal adrenal tissue then develops into three distinct secretory layers, each with different cell types. Surrounding the adrenal medulla is the zona reticularis, responsible for the synthesis of adrenal androgens including dehydroepiandrosterone (DHEA) and hormone precursors to testosterone and estrogens.
DHEA has been reported to notably increase insulin sensitivity in the pancreas and the liver and is a likely candidate for supplementation in the diets of those with diabetes mellitus. Based on trials utilizing animals, administering DHEA to animals has been shown to prevent the onset of diabetes mellitus. In a study of twenty-eight women conducted, after twelve weeks of dosing of DHEA showed fasting insulin and glucagon were lower, suggesting a possible aid in diabetes. However, further research must still be done on this topic
The second layer of the adrenal cortex is the zona fasciculata, responsible for glucocorticoid synthesis. Glucocorticoids, like corticosterone and cortisol, are also known as “stress hormones”. As a natural anti-inflammatory hormone, glucocorticoids have the ability to inhibit B cells, T cells, and phagocytes, leading to increased risk of infection, osteoporosis, and weight gain when in excess. However, they can also be used to treat various neurological diseases, such as multiple sclerosis or other demyelinating central nervous system diseases. The outermost cortical layer, encased by the adrenal capsule, is the zona glomerulosa, which produces mineral corticoids, such as aldosterone. Mineralocorticoids promote sodium reabsorption, followed by passive water reabsorption under the renin-angiotensin system (RAS). [See Part 5 – Blood Pressure and Volume for more on the RAS.]. The adrenal portal system connects capillary beds in the layers of the adrenal cortex to those found in the medulla; blood flows from cortex to medulla. This adrenal portal system is similar to the hypophyseal portal system connecting the hypothalamus to the anterior pituitary. When corticosteroids, which are steroid hormones that primarily contribute to the maintenance of resting and stress-related homeostasis, are released by the zona fasciculata of the adrenal gland, they travel through the adrenal portal system. From there, they travel through the adrenal medulla, before reaching systemic circulation. The adrenal medulla has glucocorticoid receptors, activation of which amplifies catecholamine release in response to sympathetic innervation. In other words, the sympathetic response is enhanced in the presence of HPA (hypothalamic pituitary adrenal) axis activation. The positive feedback between the HPA axis and the sympathetic response is the reason that stressors that are not necessarily physical danger can create the same sympathetic responses that imminent danger can. Additionally, continuous activation of the sympathetic nervous system because of circulating glucocorticoids can lead to long term health defects.
HPG Axis
In addition to the HPT and HPA axes, the hypothalamus also serves as a master control center for the functioning of the gonads (male testes and female ovaries) via the hypothalamus-pituitary-gonad axis (HPG axis). The hypothalamus releases gonadotropin-releasing hormone (GnRH) into the portal system with the anterior pituitary. Mature GnRH neurons release pulses of GnRH to stimulate the anterior pituitary. In response to the pulsatile release of GnRH, the anterior pituitary synthesizes gonadotropins. The excitatory effect of GnRH on gonadotropin production is primarily present when it is released in a pulsatile fashion. In fact, continuous stimulation of GnRH receptors actually prevents the excitatory effect on gonadotropin production (more on this later). There are inter-sex differences in how the anterior pituitary responds to GnRH. The gonadotropins produced and released by the anterior pituitary include follicle stimulating hormone (FSH) and lutenizing hormone (LH). Among many other roles, gonadotropins stimulate the synthesis of sex steroids by the gonads. Since sex steroids and corticosteroids both arise from the same precursor (cholesterol), the HPA and HPG axes compete for limited amounts of cholesterol for steroidogenesis.
Steroid Hormone Synthesis
Steroids are composed of four carbon rings (three six-carbon rings, and one five-carbon ring). There are multiple parallel pathways involved in steroid hormone synthesis from cholesterol precursors; glucocorticoid synthesis, mineral corticoid synthesis, and sex steroid synthesis, overlap in their initial steps. Cholesterol is converted to pregnenolone by a cholesterol side-chain cleavage enzyme in the mitochondria, removing six carbons from the 27-carbon cholesterol. Pregnenolone is a progestogen, all of which have 21 carbons. Pregnenolone can either be converted to progesterone by 3-β-hydroxysteroid dehydrogenase (3β-HSD), or can be converted to 17α-hydroxypregnenolone by 17α-hydroxylase. Both progesterone and 17α-hydroxylase can be converted into 17α–hydoxyprogesterone by either 17α-hydroxylase or 3β-HSD, respectively. These steroid hormones can, themselves, be released. Otherwise, pathways diverge, either leading to glucocorticoid and mineral corticoid synthesis, or sex steroid synthesis.
Glucocorticoid and mineral corticoid synthesis pathways demand the initial activity of 21-hydroxylase, which converts progesterone to deoxycorticosterone and 17α-hydroxyprogesterone to 11-deoxycortisol. By the action of 11β–hydroxylase in the mitochondria, 11-deoxycortisol is converted to cortisol and deoxycorticosterone is converted to corticosterone. The additional action of aldosterone synthase (which is only found in mitochondria of the zona glomerulosa), converts corticosterone to the mineral corticoid aldosterone. 11β-hydroxylase transcription is enhanced by ACTH, shifting steroidogenesis to favor glucocorticoid production (and consequently mineral corticoid production by increasing the availability of aldosterone precursors).
Without sufficient activity of 21-hydroxylase, there will be diminished production of glucocorticoids and mineral corticoids. In other words, glucocorticoid and mineral corticoid synthesis require the presence of 21-hydroxylase. Similarly, without sufficient activity of 17, 20-lyase, there is diminished production of androgens (and consequently estrogens) from progestogens. Within the adrenal cortex, androgen production occurs within the zona reticularis. 17,20-lyase converts 17α-hydroxypregnenolone to dehydroepiandrosterone, and converts 17α-hydroxyprogesterone to androstenedione. These reactions remove 2 carbons from the 21-carbon progestogens, so androgens all have 19 carbons. Dehydroepiandrosterone can be converted to androstenedione by 3β-HSD, or can be converted to androstenediol by 17β–HSD. Androstenediol and androstenedione are both precursors to testosterone. Androestenediol is converted to testosterone by 3β-HSD, and androstenedione is converted to testosterone by 17β-HSD. In certain tissues (e.g., prostate gland) testosterone can be converted to 5α-dihydrotestosterone (5α–DHT) by the activity of 5α–reductase. 5α-DHT binds to testosterone receptors with 2-3 times higher affinity, and is necessary for normal masculinization in chromosomal males.
To convert androgens to estrogens, yet another carbon is removed such that all estrogens have 18 carbons. Testosterone can be directly converted to estradiol (what is generally called “estrogen”) by the action of a generic P450 aromatase. However, androstenedione can also be converted to estradiol in a two-step process. First it is converted to an estrogen called estrone by aromatase, and then to estradiol by 17α-HSD. Estrone and estradiol can both be converted to estriol within the liver and placenta. The activity of the generic P450 aromatase is subject to regulation by dietary sources. Tofu, corn oil, peanut oil, and alcohol all enhance the activity of aromatase, leading to higher levels of estrogens at the expense of androgens.
Note that 21-hydroxylase is a necessity for the production of glucocorticoids and mineral corticoids, and that 17, 20-lyase is a necessity for the production of sex steroids. Furthermore, it is important to note that certain enzymes can catalyze different steps in multiple parallel pathways. Furthermore, aside from the cholesterol side-chain cleavage enzymes, 11β-hydroxylase, and aldosterone synthase (all of which are found in mitochondria), the remaining enzymes involved in initial steroid hormone synthesis function on the smooth ER.
In addition to catalyzing different steps in multiple parallel pathways, the same enzyme can often catalyze multiple steps within the same pathway. The enzymes involved in steroid hormone synthesis pathways are often cytochrome P450 proteins (CYP), which are monooxygenases often capable of catalyzing multiple reactions in steroidogenic pathways. For example, cytochrome P450 family 17 subfamily A member 1 (CYP17A1) is capable of both 17α-hydroxylase activity and 17, 20-lyase activity. The 17, 20-lyase activity depends on phosphorylation, cytochrome P450 oxidoreductase, and cytochrome b5. Phosphorylation of serine and threonine residues by a cAMP-dependent protein kinase increases the 17, 20-lyase activity of CYP17A1. In the absence of phosphorylation, CYP17A1 has virtually no 17, 20-lyase activity.
Since all of these synthesis pathways arise from the same limited supply of precursors, a rise in production in one type of steroid hormone is necessarily at the expense of other steroid hormones. For example, a rise in cortisol production in response to stress necessarily leads to lower amounts of androgens and estrogens. Nevertheless, the body is capable of endogenously synthesizing small amounts of cholesterol.
Steroids are lipophilic and can permeate most membranes to stimulate cytoplasmic receptors. Cytoplasmic steroid receptors generally regulate transcription. However, this is not to say that steroids don’t have membrane receptors. Since steroids are lipophilic endocrine hormones, they often require transport/carrier proteins to shield them from the hydrophilic environment of the blood. There are bound and unbound components of the steroid system. Unbound steroids can travel through the circulatory system, but they are less likely to reach their targets. Carrier proteins can also determine which receptors are activated by the steroids they carry (e.g., cytoplasmic vs. membrane receptors). Steroids typically (but not always) do not have an amplification effect; they tend to elicit effects proportional to their concentrations, but they can also have a permissive action on the activity of other signaling pathways (similar to thyroxin’s permissive action). A permissive action can either enable ligand binding to existing receptors, or it can enable expression of receptors in the first place.
Hormonal Regulation of the Male Reproductive System
Due to the differences between hormonal regulation of male and female reproductive systems, we will take a more in-depth look at each one independently, beginning with the comparatively simple hormonal regulation of the male reproductive system. Male reproductive hormone regulation is largely controlled by the hypothalamus. The pulsatile release of GnRH drives the release of LH and FSH. LH and FSH are then able to exert their effects on the male gonads, the testes, which are housed in the scrotal cavity.
The testes, along with other male sex organs, are responsible for the production, storage, nourishment, and transport of male gametes, sperm. FSH is primarily responsible for stimulating initial stages of sperm production, and LH contributes by stimulating the secretion of testosterone. In addition to its role in sperm production, testosterone also exerts negative feedback upon the hypothalamus and anterior pituitary.
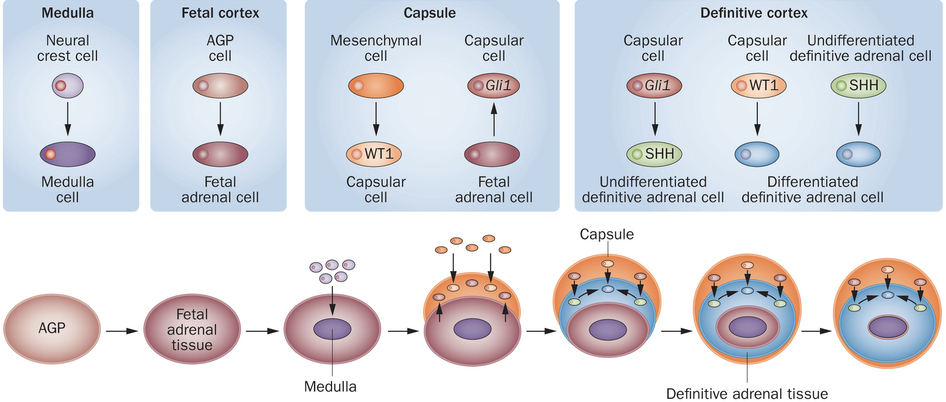
The testes are the site of sperm production and androgen synthesis (primarily testosterone), and the secondary male sex organs are involved in the nourishment and transport of sperm. The testes contain a series of finely coiled tubes called seminiferous tubules. Seminiferous tubule lobules are separated by septa. Seminiferous tubules are surrounded by peritubular myoid cells. Sperm stem cells (called spermatogonia) are at the outermost layer within seminiferous tubules, furthest from the lumen.
In the interstitial space surrounding seminiferous tubules, there are capillary beds and specific types of interstitial cells called Leydig cells. Leydig cells secrete testosterone in response to LH stimulation. Testosterone exerts negative feedback on the hypothalamus and anterior pituitary, but also masculinizes the brain, maintains libido, stimulates bone and muscle growth, establishes and maintains male secondary sex characteristics, and maintains accessory glands and organs of the male reproductive system. Testosterone secreted by Leydig cells is also necessary for the production of sperm.
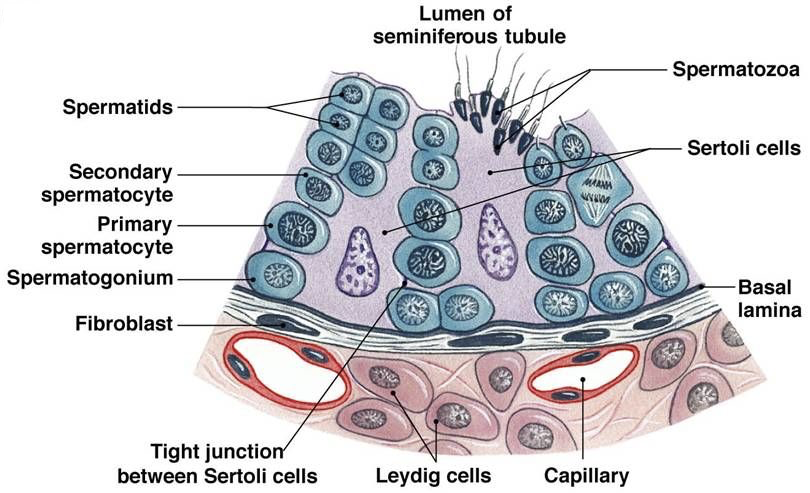
Sertoli cells (also called nurse cells) are a type of sustentacular cell that form the epithelium within seminiferous tubules. They extend from the basement membrane of the tubule all the way to the lumen. In response to FSH, Sertoli cells release androgen binding protein (ABP). The release of ABP is enhanced in the presence of testosterone (e.g., from Leydig cells). Because free testosterone is lipophilic, it is able to cross membranes. While this would normally prevent accumulation of testosterone in the seminiferous tubules to a degree necessary for efficient sperm production, ABP binds to testosterone, preventing testosterone from diffusing, and thereby concentrating testosterone within seminiferous tubules. Testosterone, concentrated by ABP in response to FSH, stimulates spermatogenesis at a rate of nearly 1000 spermatozoa produced per second.
Spermatogenesis begins with type Ad spermatogonia in the outermost layer of the seminiferous tubules. These spermatogonia are type A with a dark nuclei (type Ad). Each type Ad spermatogonium divides via mitosis into two daughter cells. One of these daughter cells remains a type Ad spermatogonium for later sperm production, whereas the other is a type Ap spermatogonium (type A with a pale nucleus). A type Ap spermatogonium divides repeatedly through mitosis, and later divide into type B spermatogonia. Type B spermatogonia divide via mitosis, yielding primary spermatocytes. Each primary spermatocyte divides into two secondary spermatocytes via meiosis I. Each secondary spermatocyte divides into two spermatids via meiosis II. Thus, each primary spermatocyte yields four haploid spermatids. With each cell division via mitosis and meiosis, cells migrate further from the basement membrane of the seminiferous tubule and closer to the lumen. Spermatids adjacent to the lumen develop into immature spermatozoa through spermiogenesis, and then enter the lumen of the seminiferous tubule.
Once an immature spermatozoon is in the lumen of the seminiferous tubule, it travels to the rete testis, which connects to the head of the epididymis. The epididymis (divided into a head, body, and tail) stores and protects spermatozoa, supports their maturation, and serves as a recycling center for any damaged spermatozoa. The tail of the epididymis connects to the vas deferens (also called the ductus deferens). The vas deferens receives excretions from the seminal glands (also called seminal vesicles). The seminal fluid accounts for about 60% of total semen volume, containing fructose for energy, prostaglandins to support sperm motility, and fibrinogen. Each vas deferens converges with an ejaculatory duct, which pass through the round, muscular prostate gland. The prostate gland excretes prostate fluid, a slightly alkaline fluid that helps neutralize urinary acids in the urethra that may otherwise damage sperm. This prostate fluid accounts for about 30% of total semen volume. Before exiting the prostate, each ejaculatory duct converges with the urethra. Immediately inferior to the prostate gland, the bulbourethral gland excretes a thick, alkaline mucus that also contributes to neutralization of urinary acids found in the urethra. At this point, semen (containing spermatozoa suspended in fluids from multiple glands) travels in the urethra through the penis. Each ejaculation typically contains about 2 to 5 mL of semen, with 20 to 100 million spermatozoa. Each of these glands requires stimulation by testosterone to maintain normal function.
In addition to their role in spermatogenesis, Sertoli cells also exert negative feedback upon the release of FSH by the anterior pituitary through the secretion of inhibin. Inhibin-mediated suppression of FSH secretion is achieved through competitive antagonism at the activin receptors. Activin receptor activation could otherwise enhance FSH secretion.
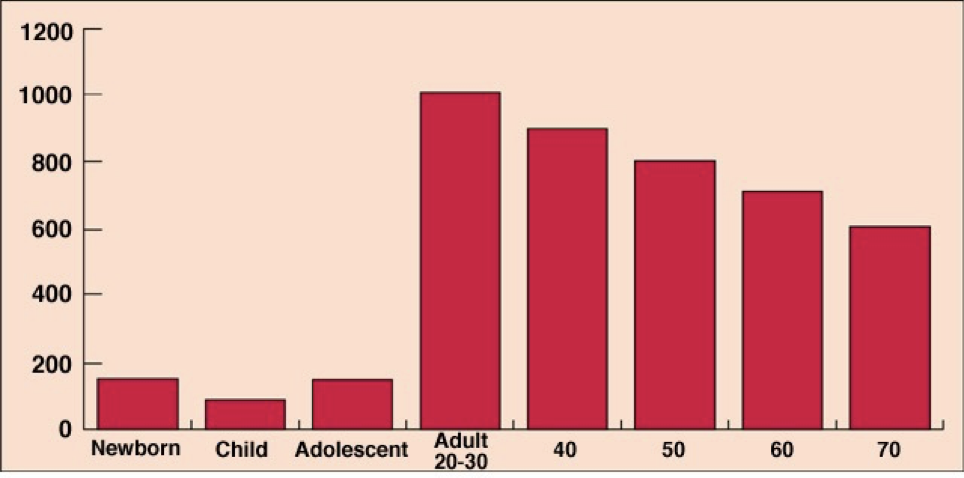
Testosterone production peaks in males between the age of 20 and 30, and then gradually declines, but remains substantially higher than any time before adolescence. The overall production of hormones involved in the reproductive cycle (GnRH, gonadotropins, androgens) remains relatively constant over the course of a month.
It is important to note that males also constitutively produce progesterone, primarily in the testes during testosterone production. Progesterone also exerts negative feedback upon the secretion of GnRH by the hypothalamus. This constant production of progesterone also provides precursors for the synthesis of other steroid hormones, such as aldosterone. Furthermore, progesterone itself can bind to mineral corticoid receptors (with a binding affinity stronger than either mineral- or glucocorticoids!). However, progesterone acts as an antagonist at these receptors and can induce natriuresis.
To maintain the optimal temperature in the testes for spermatogenesis, different muscles are involved in regulating the proximity of the testes to the body, and the surface area exposed to the external environment. The cremaster muscle is part of the spermatic cord. Upon contraction of the cremaster muscle, the cord shortens, pulling the testes closer to the body. The dartos fascia (dartos muscle) controls the surface area of the scrotum; contraction of the dartos fascia wrinkles scrotum, reducing the surface area.
Obesity and Male Infertility
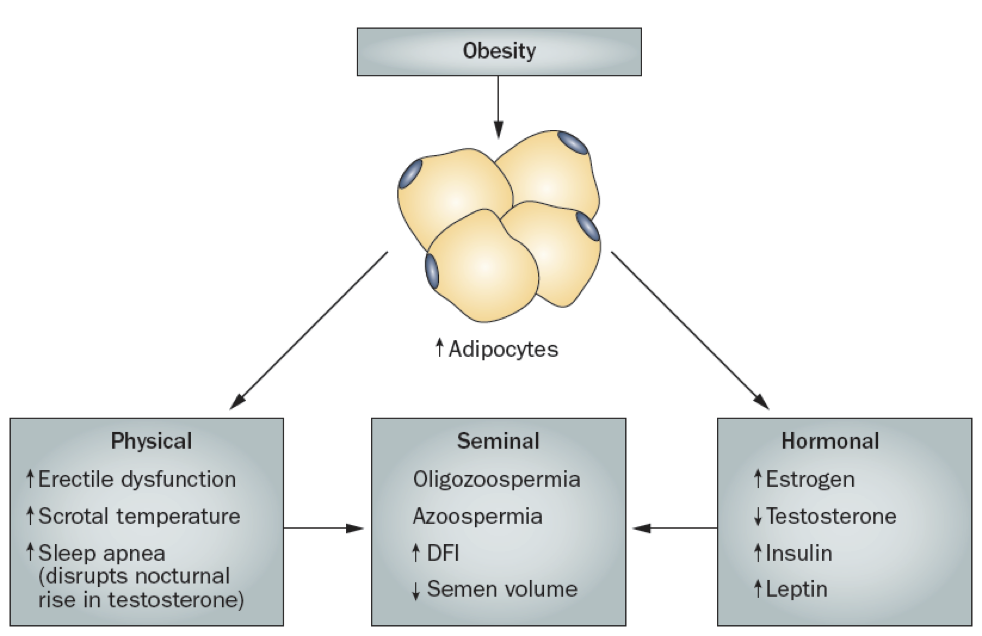
While males are often capable of producing sperm well into old age, increases in adiposity can impair fertility. The increase in adipocytes yields both physical and hormonal contributions to male infertility. There may be an increase in erectile dysfunction, increased scrotal temperature, and increased sleep apnea. The increase in scrotal temperature impairs spermatogenesis, which requires temperatures a few degrees below normal body temperature (which is why the testes are housed in the scrotum, away from the warm core).
The increased sleep apnea may disrupt the natural nocturnal rise in testosterone, preventing testosterone’s stimulation of spermatogenesis. Additionally, the increase in adipocytes also increases the prevalence of the generic P450 aromatase that converts androgens to estrogens. This leads to a net increase in estrogens and a corresponding decrease in androgens. The increase in estradiol production contributes to the inhibition of GnRH release, significantly altering the HPG axis. Furthermore, estradiol also directly inhibits spermatogenesis. Some of these symptoms, such as sleep apnea, can be treated with testosterone supplementation.
Hormonal Regulation of the Female Reproductive System
Hormonal regulation of the female reproductive system is comparatively more complex than hormonal regulation of the male reproductive system because rather than maintaining a somewhat constant hormonal environment to support the constant functioning of male reproductive organs, female reproductive organ functioning requires a cyclic change in hormones to support the multi-stage development of ovarian follicles housing the female gametes, oocytes, and to prepare the body for pregnancy in the event that the oocyte is fertilized by a spermatozoom (i.e., a male gamete). This hormone cycle can be broken into various phases around a roughly 28-day cycle, called the menstrual cycle. However, there is variation between individuals and within individuals in the actual duration of the cycle (and in the durations and qualities of phases of a single cycle). The use of a 28-day cycle is for simplicity, and merely represents a model of a generalized female reproductive cycle. The menstrual cycle includes both an ovarian cycle and a uterine cycle.
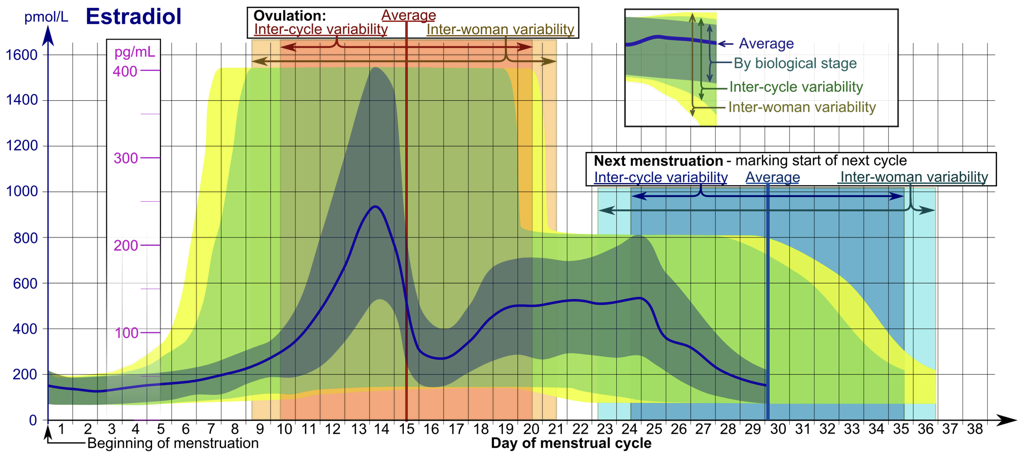
Like male reproductive hormone regulation, regulation of female reproductive hormones is largely under hypothalamic control. The hypothalamus releases pulses of GnRH to the anterior pituitary. This pulsatile GnRH stimulates the production and secretion of FSH, as well as the production (but not necessarily secretion) of LH. Again, GnRH will only excite the anterior pituitary when released in a pulsatile fashion.
The first 14 days of the 28-day menstrual cycle are called the follicular phase of the ovarian cycle, dominated by the hormonal activity of a developing follicle(s). Initially, FSH levels are higher than LH levels. While FSH in males drives sperm production, FSH in females stimulates the development of follicles, each of which supports an oocyte that is present from birth. These follicles will secrete inhibin, which exerts negative feedback upon FSH production and release by the anterior pituitary. Developing follicles will also secrete estradiol, which affects nearly every cell in the body, but notably exerts negative feedback upon the anterior pituitary and hypothalamus.
Follicle cells communicate with each other and to their oocyte with paracrine hormones. Some follicles will develop more favorable reproductive characteristics than others. In general, only a single follicle will emerge as the preovulatory follicle. However, it is possible for multiple preovulatory follicles to develop. Whereas sustentacular cells (specifically Sertoli cells) in testes produce testosterone, the production of estrogens in the female gonads (ovaries) occurs largely in developing follicles. In general, estrogens affect the central nervous system, stimulate bone and muscle growth, establish and maintain secondary female sex characteristics, maintain accessory glands and organs, and stimulate growth of the endometrium. The endometrium is the outermost layer of the uterus, exposed to the uterine environment. The uterus is divided into three layers. Adjacent to the endometrium is the muscular myometrium, which contains branches of uterine arteries. These uterine arteries in the myometrium branch into spiral arteries within the endometrium. Lastly, the innermost layer is the perimetrium.
The follicle releases estradiol, which binds to receptors on the anterior pituitary. At the beginning of the follicular phase, the follicle is only able to secrete a small concentration of estradiol, which is only able to stimulate high-affinity receptors on the anterior pituitary, called estrogen receptor α (ERα). Activation of these receptors exerts weak inhibition on the secretion of LH. At low concentrations, estradiol inhibits the anterior pituitary by binding to high-affinity, inhibitory ERα. However, estradiol does not exert significant inhibition on the production of LH, so LH stores accumulate in the anterior pituitary, driven by GnRH.
At around day 7 of the 28-day cycle, a dominant follicle is selected. These first 7 days of the follicular phase of the ovarian cycle coincide with the menses (the first stage of the uterine cycle). Menses is the destruction and loss of the functional zone of the endometrium from the previous menstrual cycle. This means that a full menstrual cycle, including ovulation, can occur before menses. In other words, pregnancy can occur before menarche (the first menses) if successful ovulation occurs before menarche.
Between days 7 and 10, the dominant follicle matures, and estradiol production continues to increase. As estradiol levels rise, the functional zone of the endometrium is repaired and regenerated. Days 7 to 14 are the proliferative phase of the uterine cycle. This entails the production of multiple tissue types within the endometrium. At the same time, FSH gradually drops due to rising levels of circulating inhibin secreted by the follicle.
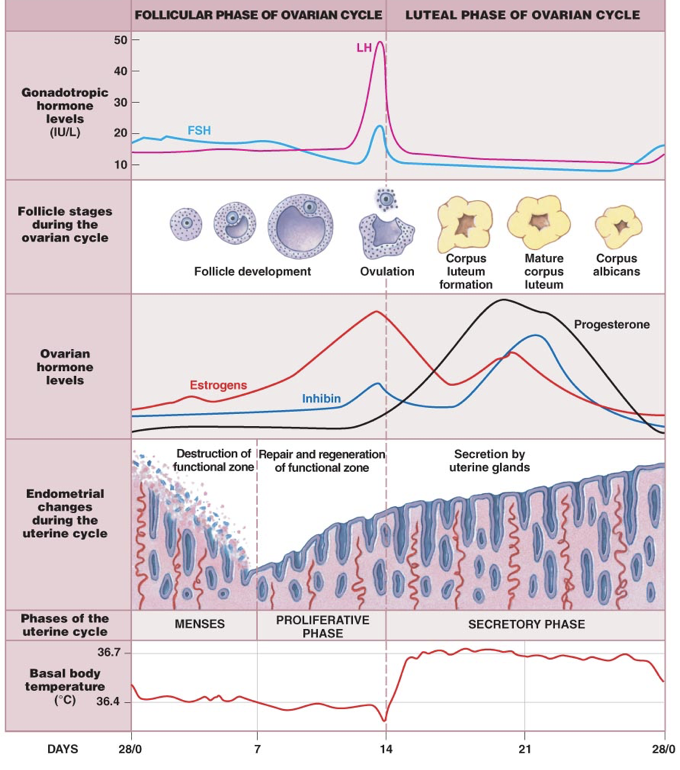
Between days 10 and 14, the endometrium continues to repair and regenerate as the follicle develops and estradiol production continues to increase. Though exerting an inhibitory effect on LH secretion, ERα stimulation induces expression of low-affinity ERβ’s (beyond their low basal expression), activation of which exerts strong excitation on the secretion of LH. Once the high-affinity inhibitory ERα’s become saturated at about day 10, estradiol can activate the newly expressed low-affinity ERβ’s, which overcome the weak inhibition from high-affinity receptor activation. This process initiates a massive secretion of LH stores from the anterior pituitary, termed the LH surge.
As follicles grow, they respond to the combination of estradiol and FSH with increased expression of LH receptors (LHCG receptors). When the LH surge occurs (generally when the follicle has become a tertiary follicle), LH drives the rupturing of the follicle, and the release of its oocyte and the corona radiata. This release of the oocyte is called ovulation, and occurs around day 14.
After this point, the rise in LH causes the remaining follicle cells to become the corpus lutuem (“yellow body”), which serves an endocrine function as it degenerates. Over the previous 14 days, the follicle increased expression of LH receptors, allowing LH to drive ovulation. Ovulation is the end of the follicular phase and the beginning of the luteal phase of the ovarian cycle. While the follicular phase is dominated by estradiol secretion by the follicle, the luteal phase (days 14-28) is dominated by progesterone secretion from the corpus luteum.
Even without implantation of a fertilized egg, the corpus luteum functions between days 14 and 25. As the corpus luteum forms from the ruptured follicle, there is a proportional increase in progesterone (specifically 17-hydroxyprogesterone) and a reduced production of estrogens. Progesterone prepares the endometrium for implantation of a fertilized egg. Whereas estradiol drives formation of the endometrium during the follicular phase, progesterone stabilizes and maintains the endometrium during the luteal phase. This includes driving angiogenesis to supply endometrial tissue with blood. There are endocrine glands in the endometrium that secrete paracrine growth hormones to prepare the endometrium for implantation. As a result, this stage of the uterine cycle, corresponding to the luteal phase of the ovarian cycle, is referred to as the secretory phase.
Progesterone also inhibits the release of GnRH, causing a drop in LH and FSH levels. However, progesterone is a relatively poor inhibitor of GnRH secretion, necessitating high progesterone levels to successfully inhibit GnRH. Progesterone in small quantities prior to ovulation (produced by the corona radiata) is insufficient to completely inhibit GnRH and reduce FSH and LH. Overall, the rise in progesterone due to growth of the corpus luteum stabilizes and maintains the body as if it were pregnant. However, if there is no implantation of a fertilized egg, the corpus luteum will degenerate between days 25 and 28. Without the progesterone from the corpus luteum to maintain the endometrium, the endometrium will begin to shed, and a new menstrual cycle will begin.
Female Infertility and Reproductive Complications
With the staggering complexity of the female reproductive system, it is miraculous that it regularly results in successful pregnancy. Like males, there are certain risks of infertility in females. One possible cause of infertility is due to endometriosis: the growth of ectopic endometrial tissue (i.e., endometrial tissue outside of the uterus). Since ectopic endometrial tissue responds to hormonal signals, it will undergo the same process of growth and degradation during the menstrual cycle. Endometrial tissue is a series of endometrial glands replaced after each menstrual cycle, necessitating rapid and frequent differentiation. If endometrial tissue ends up inside the body, the internal endometrial tissue can lead to chronic abdominal or pelvic pain, symptoms similar to IBS, interstitial cystitis, and fibromyalgia. Interestingly, pregnancy tends to prevent the future occurrence of endometriosis.
While the exact pathophysiology is unclear, there are four general theories regarding the process by which endometriosis develops. Firstly, endometrial tissue shed during menstruation may travel backwards along fallopian tubes, and deposit within the fallopian tubes, or it may move all the way through the fallopian tubes into the peritoneal cavity. This is referred to as retrograde menstruation. Secondly, endometrial tissues may move through the vasculature from the uterus and deposit elsewhere in the peritoneal cavity. This is referred to as vascular and lymphatic dissemination. Thirdly, multipotent coelomic cells in the peritoneal cavity may undergo metaplasia and become endometrial cells. Lastly, endometriosis may result from impaired immunity.
The prominent symptoms of endometriosis are often classified as the four D’s: dysmenorrhea (painful and heavy menstruation), dysuria (painful urination), dyschezia (painful defecation), and dyspareunia (painful intercourse). Endometriosis can also lead to infertility. For example, if endometrial tissue develops in the fallopian tubes, it can impinge the passage of the oocyte to the uterus, which is necessary for a successful implantation. Treatment of endometriosis generally involves the administration of hormones to mimic the hormonal state of either menopause or pregnancy, which prevent or disrupt the normal menstrual cycle. The hormones administered may be GnRH agonists, combined oral contraceptives (estrogens and progestogens), or only progestogens.
While endometriosis is the presence of extra endometrial tissue outside the uterus, the absence of endometrial tissue is also problematic and results in the absence of menstruation, called amenorrhea (amenorrhea also results from other causes). This can derive from a rather rapid drop in estrogens and progesterone, which results in a failure to produce endometrial glands. Estrogen-suppressing drugs can destabilize the endometrium and lead to spotting. Amenorrhea can result from insufficient nutrition (not enough calories), making female athletes more susceptible. It can also result from abnormally low body fat (e.g., due to anorexia nervosa or starvation), as well as hormonal imbalances (e.g., due to a pituitary tumor). Treatment for amenorrhea also involves hormone therapy.
Menopause
Infertility is inevitable at some point in life due to the depletion of oocytes, of which there are a limited number present at birth. As females age, they inevitably undergo menopause, the cessation of menstruation. Menopause is defined as the absence of menstruation for more than 12 months and presents with symptoms for a period of about four years, usually beginning in the mid- to late-40s. The development of menopause is associated with the depletion of follicles, and the consequently-impaired ability to produce sex steroids. Menopause is often associated with numerous changes throughout the body, which present as cognitive symptoms (including depression, anxiety, memory loss, labile mood, headaches), hot flashes (sudden feelings of warmth in the face, neck, and chest that last for about four minutes) and consequent night sweats, joint pain, weight gain, urinary problems (urgent and frequent urination, dysuria, incontinence), and vaginal changes (dryness and increased susceptibility to infection, discomfort, itching, dyspareunia, atrophy).
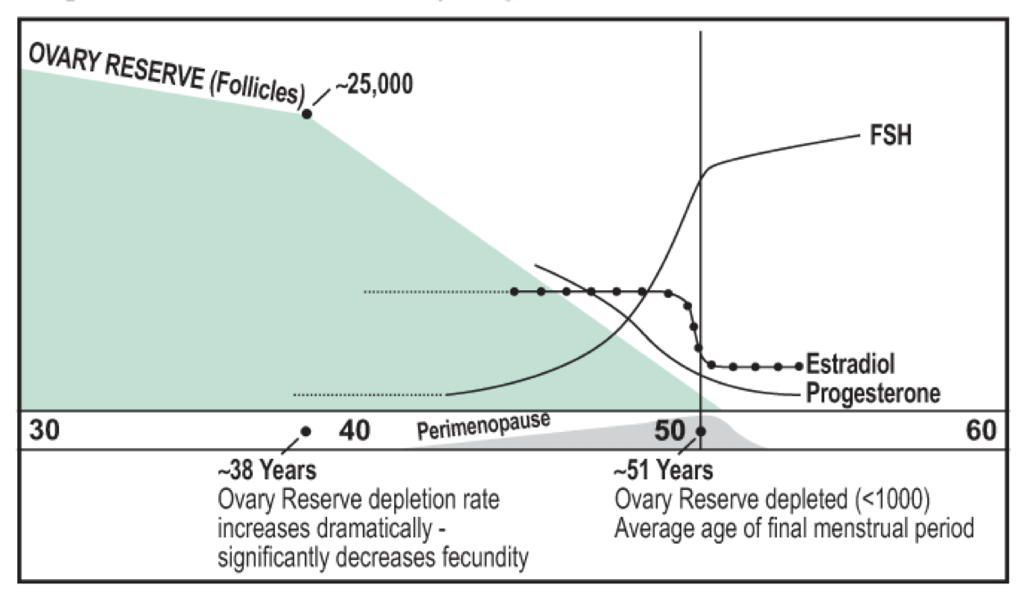
With a decreasing number of follicles, there is a decrease in estradiol and progesterone production. This removes negative feedback on GnRH secretion, leading to an increase in GnRH secretion and a consequent increase in FSH. Elevated FSH is a problem because it causes remaining follicles to grow at abnormal rates, which can precipitate ovarian cancer. Often, premenopausal (non-menopausal) females will have high FSH levels. These females typically have difficulty getting pregnant. The high level of FSH indicates low levels of progesterone and estradiol. This may be due to stress and an increase in cortisol, but in females in their mid- to late-40s, it is most likely due to a lack of follicles. Extra-gonadal FSH receptors also exist, which may explain some other post-menopausal changes in female physiology. Low estradiol is itself a problem because of estrogens’ diverse functions.
Unfortunately, there is no way to prevent menopause due to its natural physiological origin. However, the symptoms of menopause (and the hormonal changes still present during post-menopause) can be treated with hormone therapy, usually with estrogen supplementation. Estrogen supplementation is particularly important because a significant consequence of low estrogen levels is the risk of losing bone mass and density, which results in osteoporosis (porous bones).
Estrogens play an important role in bone health. Estrogens affect osteoblasts and osteoclasts, largely through their impact on nuclear factor κB (NFκB) signaling pathways. NFκB is a family of transcription factors, activation of which occurs in response to a wide range of stimuli, including cytokines, immune modulators, and other stressors; NFκB activation is a downstream outcome in certain glucocorticoid-activated pathways (e.g., cortisol). In bones, NFκB mediates the production of cytokines that inhibit osteoblast differentiation and mineralization, while promoting osteoclast differentiation. In fact, activation of NFκB is required for osteoclast differentiation. Estrogen receptor (specifically ERα) activation blocks the NFκB pathway in osteoblasts. This action of estrogens promotes osteoblast differentiation and survival. Furthermore, estrogen receptor activation in osteoblasts suppresses the production of receptor activator of NFκB ligand (RANKL) and increases production of osteoprotegrin (OPG). Normally, RANKL binding to RANK on osteoclast precursors activates the NFκB pathway, driving osteoclast differentiation in bones (RANKL binding to RANK is just one of many interactions that activates NFκB). Suppressing the production of RANKL reduces activation of RANK. Increasing production of OPG (which binds RANKL to prevent its activation of RANK) further reduces activation of RANK. As a result, estrogen-mediated decreases in RANKL and increases in OPG inhibit osteoclast differentiation and limit subsequent bone loss.
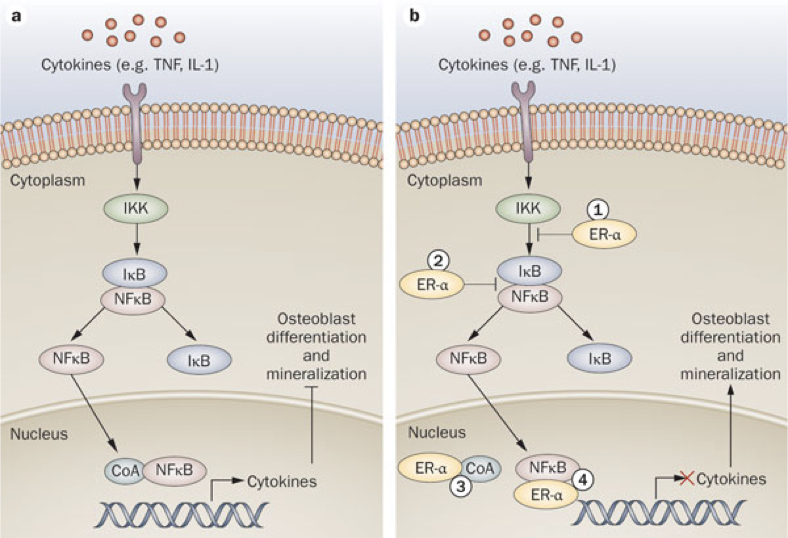
Osteoporosis occurs more often in females than males due to their loss of estrogens during menopause, which contributes to a net decrease in osteoblast activity and increase in osteoclast activity. However, any periods that increase stress (and therefore cortisol synthesis) will not only shift the balance of osteoblast activity to favor a loss of bone mass, but corresponding increases in cortisol also entail diminished cholesterol precursors available for estrogen synthesis. In other words, stress can also lead to a decrease in estrogen levels and consequent detriment to bone health.
Estrogens are used to treat osteoporosis, IBS, and skin disorders, while also reducing susceptibility to ovarian cancer through suppression of FSH. However, estrogen treatments can also increase the risk of thrombosis. One of estradiol’s hepatic effects is to inhibit expression of hepcidin. With excessive estrogens, there is abnormally low hepcidin, which can lead to blood clots. In contrast, the absence of estrogens and consequent increase in hepcidin expression contribute to the development of osteoporosis, iron accumulation in tissues, and low iron availability for red blood cell production.
Manopause
Similar to females that undergo menopause, males undergo “manopause,” a gradual drop in testosterone. This commensurate change in males also leads to a rise in FSH, which also leads to age-related changes. However, the degree of testosterone loss in males is less severe than the degree of estrogen loss in females. Nevertheless, the loss of testosterone can also lead to osteoporosis. In fact, if the degree of testosterone loss in males were comparable to the degree of estrogens lost in females, there would be a greater prevalence of osteoporosis in males than females.

Feedback/Errata