12 Sleep and Circadian Neuroendocrinology
Sleep and Circadian Neuroendocrinology
Maintaining homeostasis necessitates time for repair and growth processes to occur. These processes are largely influenced by the hypothalamus and are most prevalent during sleep. In fact, the hypothalamus is as active during sleep as it is during periods of wakefulness. Growth hormones are secreted during sleep to initiate signaling cascades that drive cell/tissue repair, proliferation, differentiation, and growth. Importantly, people under high levels of stress do not release growth hormones in a normal way, and generally fail to benefit from restorative aspects of sleep to the same degree as non-stressed individuals.
One of the most important processes that occurs during sleep is memory consolidation. In fact, most memory consolidation appears to occur during sleep. The hippocampus requires sleep for memory consolidation. Interestingly, hippocampal firing patterns measured during a location-training task are repeated during sleep but in a reversed, condensed sequence. Sleep also appears to be the period wherein memories stored in the hippocampus are distributed to other regions (e.g., frontal/parietal lobes) for long term retention.
In addition to its roles in regulating the processes that occur during sleep, the hypothalamus also regulates the initiation of sleep itself. Almost all physiological processes exhibit rhythmic patterns. Some rhythms have yearlong cycles (e.g., hibernation) whereas others can be very short (e.g., a heartbeat). Within the brain, there are numerous types of biological rhythms generated that are different in quality, duration, and function. Among these many biological rhythms, two important rhythmic processes in the brain are rapid electrical rhythms, generated by the summated activity of thousands of neurons, and circadian rhythms, which regulate numerous processes that cycle over the course of a day, including sleep/wake cycles.
Circadian rhythms are biological processes that oscillate over a period of about 24 hours and are under the control of a “circadian clock.” Hormone production and release rates, body temperature, cardiovascular control, renal filtration rate, and metabolic rates all rise and fall predictably according to circadian rhythms. Though biological clocks can independently and consistently coordinate oscillations in physiological processes, they are subject to modulation by environmental cues.
The suprachiasmatic nucleus (SCN), in the anterior hypothalamus above the optic chiasm, serves as a dominant circadian clock. It is capable of operating without sensory input to maintain a nearly 24-hour cycle and controls physiological processes according to its timing. However, in the absence of environmental cues, the cycle tends to lengthen to about 24.5 hours, resulting in a delayed circadian cycle. A delayed circadian cycle is a circadian cycle that begins and ends later than would be expected under the local light cycle (i.e., hormone release each day is delayed). This leads to a difficulty falling asleep until late in the night, which necessitates wake up times later in the morning or early afternoon. In contrast, an advanced circadian cycle begins and ends before the normal light cycle, leading to earlier bed times and earlier wake up times.
Neurons in the SCN undergo a roughly 24-hour long molecular negative feedback cycle based on gene expression, wherein the product of clock and BMAL1 genes (called clock and cycle in drosophila) gradually increase in concentration until the products of their cellular pathways are capable of inhibiting the transcription of clock and BMAL1 production. This causes the concentration of clock and BMAL1 to drop, and the cycle repeats.
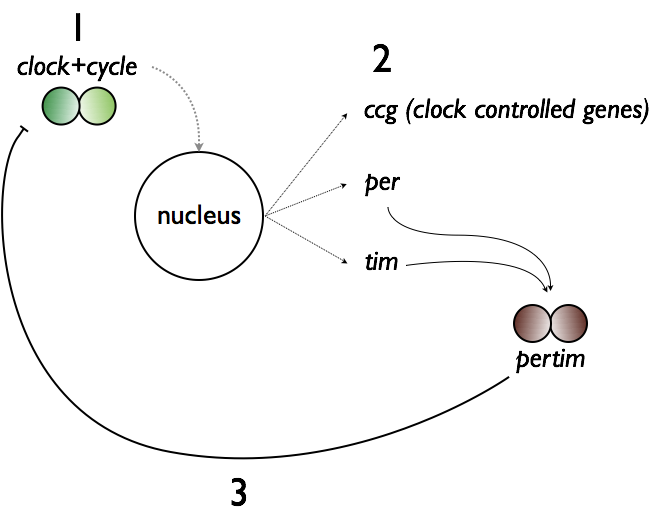 Transcription of clock and BMAL1 genes drives the transcription of clock-controlled genes (ccg), as well as period and cryptochrome, which yield per and cry (called per and tim in drosophila). Per and cry dimerize and subsequently impair transcription of clock and BMAL1 genes before degrading. This SCN gene cycle is thought to be the
Transcription of clock and BMAL1 genes drives the transcription of clock-controlled genes (ccg), as well as period and cryptochrome, which yield per and cry (called per and tim in drosophila). Per and cry dimerize and subsequently impair transcription of clock and BMAL1 genes before degrading. This SCN gene cycle is thought to be the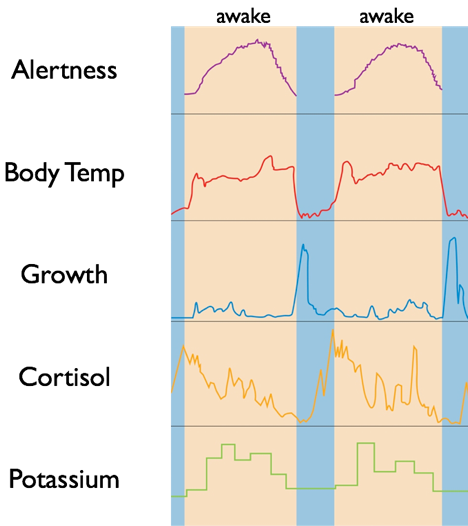 mechanism responsible for establishing the dominant central circadian rhythm. Because each SCN neuron’s genetic cycle may operate at slightly different rates, chemical synapses, gap junctions, and participation of glia help SCN neurons synchronize their activity and provide a uniform signal of time. In fact, nearly every cell in the body has a similar type of negative feedback loop that drives its own circadian clock, but the timing of these clocks is aligned by the dominant SCN.
mechanism responsible for establishing the dominant central circadian rhythm. Because each SCN neuron’s genetic cycle may operate at slightly different rates, chemical synapses, gap junctions, and participation of glia help SCN neurons synchronize their activity and provide a uniform signal of time. In fact, nearly every cell in the body has a similar type of negative feedback loop that drives its own circadian clock, but the timing of these clocks is aligned by the dominant SCN.
SCN cells communicate circadian rhythms mainly through oscillating amounts of GABA-mediated innervation of nearby hypothalamic regions, other parts of the diencephalon, and midbrain structures. One of its most important contributions to sleep and wakefulness is through its inhibitory connections with the ventrolateral preoptic nucleus (VLPO). The VLPO regulates the activity of arousing histaminergic neurons of the tuberomammillary nucleus (TMN) through inhibitory, GABAergic connections. Thus, the SCN’s inhibition of the VLPO releases inhibition of the TMN, permitting the activation of histaminergic neurons projecting throughout the brain and brainstem that promote wakefulness.
The SCN’s diverse projections also permit an influence on the autonomic nervous system’s regulation of certain peripheral systems, including core body temperature maintenance, adrenal hormone secretion, and metabolism. In turn, these physiological changes caused by the SCN regulate other circadian clocks, giving the SCN indirect control of the body’s circadian rhythm. For example, under the influence of the SCN, body temperature undergoes a sharp 1°C drop each night. This serves to align daily cycles of internal organs. The time at which this 1°C drop occurs also provides an indication of circadian timing such that changes in the time of the 1°C drop indicate changes in the timing of circadian rhythms.
Central vasoactive intestinal peptide (VIP) has an important role in the regulation of circadian rhythms. VIP releasing neurons, and neurons expressing VIP receptors, are found in the ventrolateral suprachiasmatic nucleus of the hypothalamus. Neurons in the ventrolateral suprachiasmatic nucleus (those containing VIP and/or VIP receptors) receive retinohypothalamic projections, providing these neurons with signals of environmental light conditions. These neurons can then co-release VIP and γ-aminobutyric acid (GABA), which helps synchronize the suprachiasmatic nucleus to the light-dark cycle.
It is possible for circadian rhythms to drift out of a perfect 24-hour cycle, and they regularly do, but light-sensitive input pathways from the retina help entrain the SCN to the daily 24-hour light cycle (i.e., light in the day, dark at night). In turn, the SCN can synchronize circadian clocks throughout the body. The light-input pathways arise from a subset of retinal ganglion cells that are capable of transducing light using the biological pigment melanopsin, while also being able to integrate neural signals arising from traditional rod and cone pathways. These intrinsically photosensitive retinal ganglion cells (ipRGCs) project to non-image-forming brain centers to induce changes in circadian timing, changes in arousal levels and sleep drive, and changes of melatonin production (i.e., suppression of melatonin production during light exposure). Sixty percent of ipRGC fibers project directly to cells of the SCN via the retinohypthalamic tract, allowing the SCN to perceive the luminance of light stimuli, and adhere to the daily light cycle.
Per degrades in the presence of light, which destabilizes the per-cry dimer, releasing inhibition on clock and BMAL1 gene expression. Even though the SCN is the dominant circadian clock, temporary desynchronization can occur, often due to a change in the light cycle following travel or exposure to artificial light. Furthermore, the timing of the SCN can be deregulated by stress.
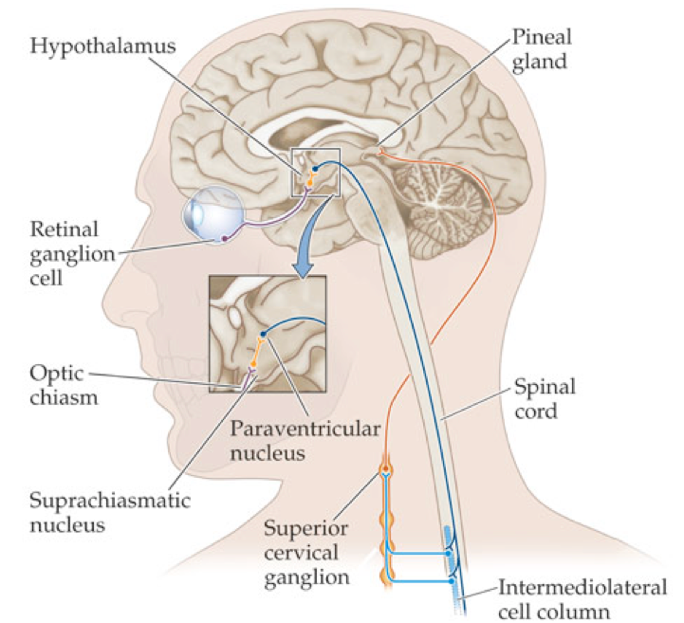 In addition to the 1°C temperature drop as a means of measuring circadian timing, under most circumstances, the timing of the SCN can be inferred by determining the time of dim light melatonin onset (DLMO), generally by analyzing measures of salivary/plasma melatonin or by measuring melatonin metabolites in urine. Melatonin is an endogenous hormone that is produced and secreted by the pineal gland under the influence of the SCN and autonomic ganglia. However, certain non-human organisms have light-permeable skulls such that the pineal gland can directly respond to the presence of light.
In addition to the 1°C temperature drop as a means of measuring circadian timing, under most circumstances, the timing of the SCN can be inferred by determining the time of dim light melatonin onset (DLMO), generally by analyzing measures of salivary/plasma melatonin or by measuring melatonin metabolites in urine. Melatonin is an endogenous hormone that is produced and secreted by the pineal gland under the influence of the SCN and autonomic ganglia. However, certain non-human organisms have light-permeable skulls such that the pineal gland can directly respond to the presence of light.
Melatonin secretion generally aligns with the SCN clock such that at night, melatonin will be secreted for about 10 to 12 hours, peaking in the early morning and falling before waking. Melatonin secretion is a general indication that the SCN perceives “nighttime.” However, it is possible for the circadian clock to shift its timing independently of changes in melatonin secretion under certain conditions.
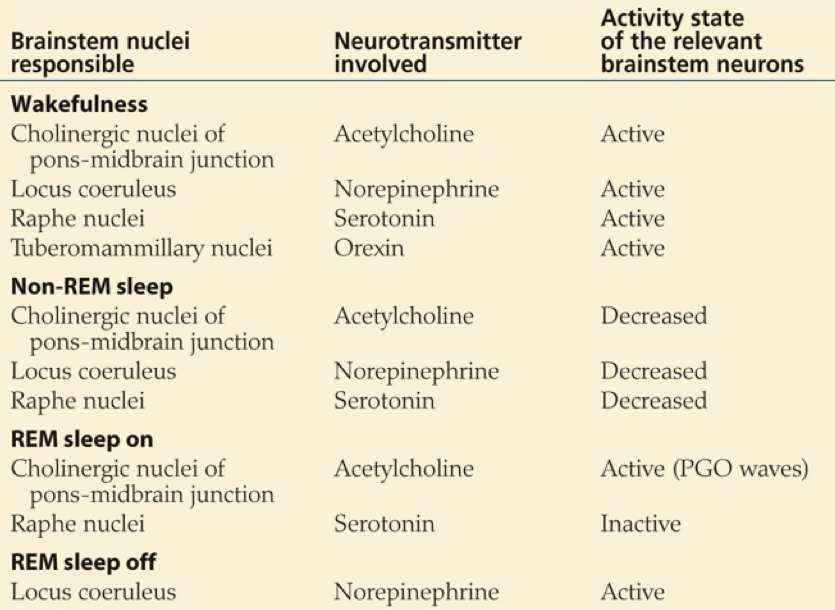
Another important transmitter involved in sleep is orexin, also called hypocretin. Orexin producing neurons of the lateral hypothalamus and posterior hypothalamus contact diverse targets throughout the brain and spinal cord. These neurons exert an arousing, wakefulness-promoting effect through stimulation of norepinephrine release by the locus coeruleus, serotonin release by the raphe nucleus, ACh release by the LDT/PPT, dopamine release by the VTA, and especially histamine release by the tuberomammilary bodies of the hypothalamus.
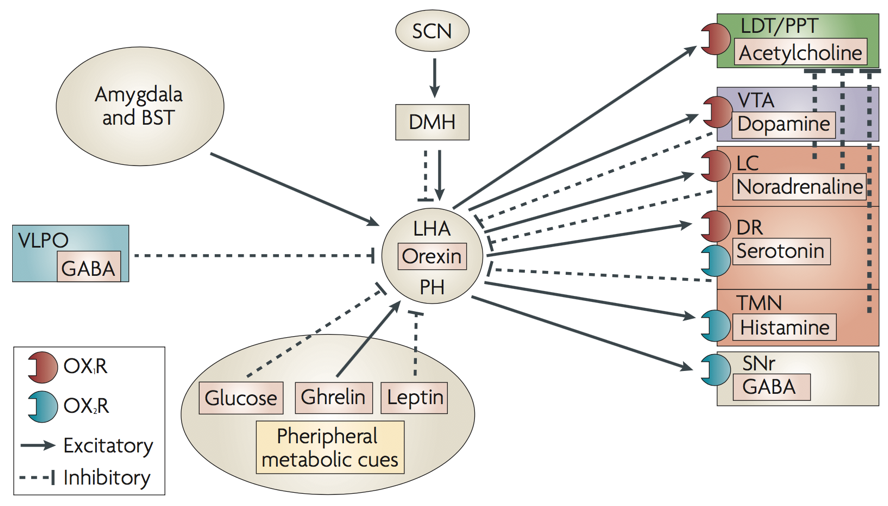
Additionally, orexin neurons from the hypothalamus modulate the reward system through their connections to the ventral tegmental area (VTA), and integrate leptin, ghrelin, and glucose signals of energy homeostasis (among many other functions). The orexin system is also under the influence of limbic structures such as the amydgala, which appears to alter orexin’s influence on arousal based on the current emotional state. For example, mild stress appears to maximally activate lateral hypothalamic orexin neurons, whereas low stress or high stress will yield underactive orexin neurons. The degree of stress is conveyed to the LHA by the magnitude of amygdalar inputs.
Melatonin’s mode of action appears to be through an inhibitory influence on the SCN. By inhibiting the SCN, melatonin prevents the SCN’s inhibition of the VLPO. This enables the VLPO’s inhibition of the TMN, ultimately decreasing histaminergic signaling. Signals that increase histaminergic signaling by the TMN, such as orexin from the LH, promote wakefulness, whereas inhibition of histaminergic signaling by the TMN, such as GABAergic inhibition from the VLPO, will drive sleep.
While certain drugs, transmitters, and hormones may promote sleepiness, slight deviations in the concentration of orexin can initiate sleep. In fact, the process of moving from a wakeful state to sleep can occur in just two seconds; without the signal to maintain wakefulness, sleep can occur rapidly. This occurs in certain disease states in which orexin receptors are absent, or there is an inability to produce orexin. The result is often narcolepsy. However, GABAergic inhibition of the lateral and posterior hypothalamic nuclei by the ventrolateral preoptic nucleus of the hypothalamus may also induce sleep. Sleep-promoting drugs, such as benzodiazepines and antihistamines, tend to exert their effects by hampering the histaminergic output of the TMN. Benzodiazepines enhance GABAergic inhibition of the TMN, and antihistamines serve as antagonists at histamine receptors, preventing the TMN from driving wakefulness.
Sleep is a readily reversible state of reduced responsiveness to, and interaction with, the environment. There are two generalized states of sleep: rapid eye movement sleep (REM sleep) and non-REM sleep, with non-REM sleep being subcategorized into 4 stages that collectively account for about 75% of total sleep time. Different stages of sleep are thought to serve different functions.
The rapid fluctuations in algebraically summated electrical activity (EPSPs/IPSPs) in the cerebral cortex can be measured from the surface of the skin with an electroencephalogram (EEG). With noninvasive surface electrodes placed around the scalp, microvolt fluctuations can be recorded in the regions between pairs of electrodes, providing an indication of the regional activity of thousands of pyramidal neurons. Voltage fluctuations must be able to pass from cortical neurons through multiple non-neural layers, the skull, and skin before reaching surface electrodes, so EEGs only record the voltage generated from large populations of neurons.
Clearly, EEG recordings have multiple limitations. Due to the reliance on large populations of neurons, an EEG really only reflects the synchronization of neural activity. In other words, a low amount of synchronous activity may generate lower-frequency, larger-amplitude fluctuations than a high degree of unsynchronized activity. Activation of all neurons in a population may yield higher-frequency, lower-amplitude fluctuations. This limitation complicates the interpretation of EEG data.
Magnetoencephalography (MEG) is an alternative to EEG that measures the magnetic fields generated by electrical activity in neuron populations. While MEG is able to localize sources of electrical activity with higher acuity than EEG, and can record electrical activity from deeper brain structures, MEG also has significant limitations. Firstly, it is challenging to detect the extremely tiny magnetic fields produced by neural activity. This is further complicated by the powerful electric field generated by the earth, and the ambient electrical fields generated by electrical current (e.g., those generated in nearby power lines, moving cars, or even elevators in the building).
Due to the limitations of EEG analysis, it is unlikely to be a reliable indication of what a person is thinking. However, it is a useful metric of the degree to which someone is thinking in targeted cortical regions. In other words, using EEG data, one can infer the intensity of information processing, but not the type of information being processed.
Low-frequency high-amplitude EEG recordings indicate stages of non-dreaming sleep, drug intoxication, and coma. Such low-frequency high-amplitude rhythms indicate that targeted regions are not involved in complex processing, but rather are phasically stimulated by a central pacemaker (e.g., the thalamus) or through intrinsic excitatory-inhibitory connections that lend to automatic synchronization in lieu of input. On the other hand, high-frequency low-amplitude EEG recordings indicate that targeted neurons are all engaged in slightly different aspects of a multifaceted complex process, preventing the summation of simultaneous activity. This can reflect an active awake mind, or dreaming stages of sleep, primarily REM sleep, in which EEG recordings are somewhat reminiscent of wakefulness. The varying levels of synchronization can result in EEGs as low as 0.05 Hz, and as high as 500 Hz (though only for brief periods). Different ranges of frequencies are denoted by different Greek letters: delta (<4 Hz), theta (4-8 Hz), alpha (8-12 Hz), sigma (12-16 Hz), beta (16-30 Hz), and gamma (30-45 Hz).
Sleep normally begins with a period of non-REM sleep. Parasympathetic nervous system (PSNS) activity dominates, resulting in decreased heart rate, respiration, kidney filtration, energy consumption, and body temperature, but an increase in digestion. The body remains largely immobile, with brief movements only to adjust body position. Brain processes reach a daily low, as indicated by slow wave, high amplitude EEG and decreased energy use.
Upon falling asleep, a healthy adult first enters stage 1 non-REM sleep, which is characterized by waning alpha rhythms and slow rolling eye movements. After just minutes, stage 1 will transition into stage 2, in which occasional sleep spindles (brief 8-14 Hz oscillations) are generated by the thalamus, and eye movement ceases. The body then drifts into stage 3, displaying delta rhythms, and then into stage 4, also called deep sleep or slow-wave sleep, in which delta rhythms drop to 2 Hz or less.
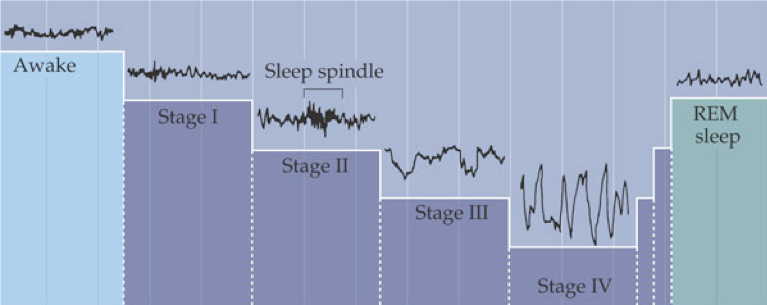
For the first cycle, the body remains in stage 4 sleep for about 20-40 minutes before returning to stage 2 or 3. After a brief period in stage 2 or 3, REM sleep begins. The sympathetic nervous system (SNS) is engaged during REM sleep, resulting in increased heart rate and respiration rate (though with increasing irregularity) and a core temperature drop. During REM sleep, glutamatergic output from the pons to the medulla is inhibited by NE and serotonin, blocking motor pathways and causing an almost total loss of skeletal muscle tone. Ideally occurring at the same time, sensory inputs from the periphery are also inhibited.
The movement-suppressing effects that occur during sleep may arise prior to loss of consciousness, leading to a perception of paralysis. In contrast, if sensory input is inhibited before motor pathways are blocked, hypnic jerks may be generated subconsciously as an intrinsic mechanism of reassuring motor control functionality.
Despite the inhibition of motor pathways controlling skeletal muscles during REM sleep, the muscles of the eye (and ear) become extremely active, along with the brain. Bursts of rapid eye movement are a strong indicator of dreaming. The active mind is also reflected by beta and gamma rhythms and increases in energy consumption, which are comparable to an active awake mind. However, it appears that dreaming begins during stage 4 (deep sleep), during which brain activity is at its lowest. In fact, waking during a dream that occurs in stage 4 permits recollection of the dream, whereas dreams that occur during REM are unlikely to be remembered when a person is awakened during REM sleep.
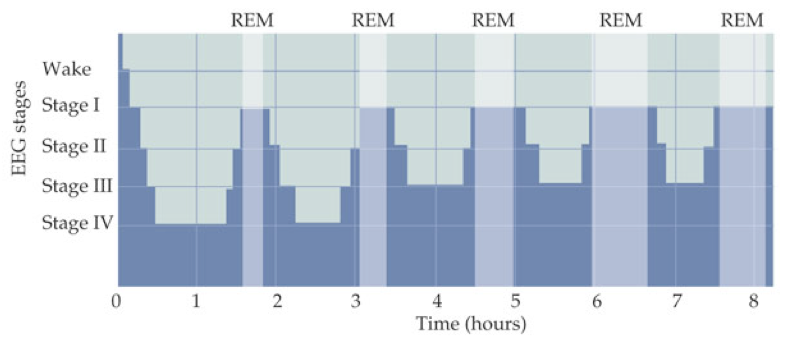
This constitutes one sleep cycle lasting about 90 minutes. Subsequent sleep cycles follow the same progression of stages but with increased REM sleep duration and decreased non-REM sleep duration.
One of the most important roles of REM sleep is its promotion of memory consolidation, though the specific mechanisms by which REM contributes to memory formation remain somewhat elusive. However, it appears that REM sleep is primarily implicated in consolidation of non-declarative memories (i.e., associational and non-associational memories; procedural memories), whereas stage 4 (deep sleep) is more strongly implicated in the consolidation of declarative memories (i.e., memories of facts and events). Sleep apnea, usually due to a problem with the vagus nerve, interferes with normal breathing and can prevent the body’s ability to enter REM sleep. This can precipitate cognitive impairments resulting from deficient memory consolidation.
It has been commonly inferred that dreaming, in some way, contributes to these memory-enhancing effects of REM. However, the dreaming that occurs during slow wave sleep is characterized by activity in distinctly different areas than those active during memory consolidation. Nevertheless, the question remains open. It is just as likely that dreams are a product of random associations unnecessary for memory consolidation.

Feedback/Errata