Understanding Paleoproxies using Ice Cores
Ashley Maloney
Overview
Focus Questions
- How do we know about Earth’s climate before instrumental records (e.g. weather stations, ship board and buoy measurements) ?
- What is a proxy?
- What are the timescales of cycles and processes captured in ice core data?
- What caused the cyclic changes in CO2 and temperature over the last 800,000 years?
- What are the two main differences between patterns and causes of natural CO2 variability during the past 800,000 years and recent CO2 levels recorded by modern instruments since the start of industrialization?
Learning Goals
Students should be able . . .
- To explain the difference between tectonic, orbital, and anthropogenic timescales.
- To use marbles as a proxy for isotopes and temperature in ice cores to understand how ice cores are used in scientific investigations of paleoclimate .
- To generate CO2 and isotopic data from ice cores that illustrate how the two variables naturally co-vary during glacial-interglacial cycles.
- To use scientific data to explain variability in the rate and magnitude of change associated with in anthropogenic emissions of CO2 and naturally cycling CO2.
Background Information for Students
Earth’s climate was quite different during the Last Glacial Maximum about 20,000 years ago when ice sheets were at their full extent. Sea levels were about 120 meters (400ft) lower, much of the Northern Hemisphere was covered in ice sheets, woolly mammoths, giant sloths, and saber-toothed cats still roamed, and the climate was cooler and drier. We learn a great deal about past climates by examining paleoproxies, and students may already be familiar with the idea that ice cores, tree rings, speleothems (cave deposits), marine sediments, lake sediments and coral skeletons can all store information about the past.
Ice cores come from every place in the world that ice accumulates over time, including tropical glaciers (see Kump et al. chapter 6 pp. 113 – 117 for glacier and ice sheet information). The most famous ice cores are from the Greenland and Antarctic ice sheets. Trapped within layers of ice are bubbles that preserve samples of the atmosphere from the past, and isotopes of hydrogen and oxygen in the frozen water that help us reconstruct past temperature.
The longest and most robust records of atmospheric CO2 are from Antarctica and the longest ice core record collected by scientists extends back 800,000 years. The CO2 record comes from gas trapped in the ice: atmospheric gases diffuse into the top layer of snow. As the top layer (“firn”) densifies it becomes ice, trapping the gas in bubbles. Old ice far below the surface has bubbles that were trapped thousands of years ago – leaving scientists with a way to directly measure Earth’s past atmosphere.
Less straightforward is the temperature record inferred from the stable hydrogen or oxygen isotopic composition of the water molecules that make up ice. Variations in temperature through time cause variations in the deuterium/hydrogen (D/H) ratios in ice. Isotope data is expressed using “delta” (δ) notation. Very negative δDice values represent colder temperatures (glacial periods) and less negative values are found during warmer climate conditions (interglacial periods).
Background Information for Teachers
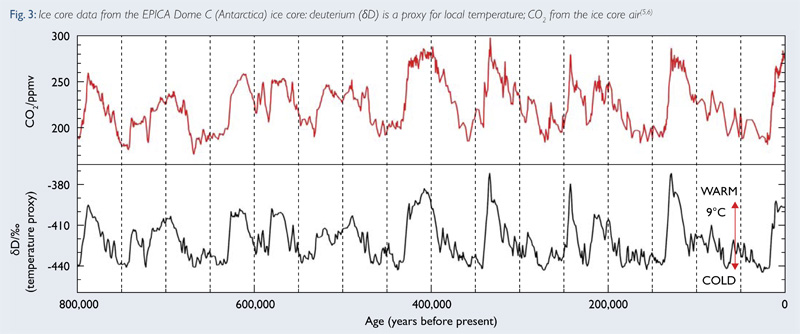
This ice cores and data in this lab are modeled off the longest ice core record available to date: EPICA Dome C, Antarctica (record shown above). The homemade ice cores you will construct for your students represent just the last 20,000 years as it is impractical to construct the entire ice core in your freezer. There are ~10 CO2 and ~80 isotope measurements for each 1000 years of ice. For the purposes of this lab CO2 concentrations and isotope date are averaged every 1000 years.
You will find all data required for this lab on six tabs of the Excel spreadsheet linked under Materials. The procedure section along with the Excel spreadsheet should provide the information needed to run the lab.
Years Before Present (yrBP) means years before 1950 as the radiocarbon standard was set to a date proceeding excessive carbon produced during nuclear bomb testing. CO2 units are PPMV = parts per million volume which reflects the concentration of CO2 in an air sample.
Image from British Antarctic Survey
Materials
- Reference textbook: Kump LR, Kasting JF, Crane GC (2010) The Earth System. Pearson (optional)
- To make ice cores
- Freezer space for five 1 ft tall tubes
- Hacksaw or pipe-cutter to cut the acrylic tube into 5 sections
- Order plastic tube ($17.22 for 6ft) at http://www.usplastic.com/catalog/ite m.aspx?itemid=32520&catid=440
- Order endcaps ($1.85 for 5 caps) at http://www.usplastic.com/catalog/ite m.aspx?itemid=29228&catid=841
- Electrical tape to attach endcaps
- Waterproof sharpie
- Green, purple, and orange marbles
- Write-in-the-rain paper
- To dissect ice cores
- Large cutting board(s) or dish bins
- Hacksaw(s)
- Hammer and chisel(s)
- Hot water
- Strainer
- 10-20 dish towels
- 20 small Dixie cups
- Work gloves and safety goggles
- Files
- Excel Spreadsheet for instructors – request access from uwpcc@uw.edu
- Worksheets (.docx) – for student
Procedure
Pre-lab (allow 1-2 weeks for preparations)
This lab is designed for five student groups (but can accommodate six). It takes a week to assemble all the material and 4 days to make the ice cores since each layer needs a day to freeze. You will make 20,000 years worth of an ice core in 5 sections. Each section has four layers and every layer represents 1000 years. The CO2 and temperature proxy data you need for each layer are on the first sheet of the Excel file (called “Making Ice Cores”) and shown in two figures below. The second sheet of the Excel file (called “Understanding the Data”) explains how the raw data were manipulated to create the twenty CO2 and twenty temperature proxy data points needed for making the ice core.
- Assemble all materials and plan out the ingredients for each layer for the 5 cores.
- Label all 5 tube-sections with dates using electrical tape and sharpie. For instance, the first tube section for Group #1 should have four layers labeled 0-1000 yrBP, 1000-2000 yrBP, and so on.
- Tape the end cap on the tube and pour in ~½ cup of water (it is better to add more rather than less water so that students have enough ice to hold on to during the dissections).
- Drop in a piece of Write in the Rain paper with the CO2 concentration written on it.
- Add the marbles that represent the hydrogen isotope data (marbles work best so they all settle in the same place whereas beads or beans tend to float and this can lead to students attributing data to the wrong layer).
- After water and marbles are added, drop in a piece of wax paper cut to fit the diameter of the tube (this step is optional but it helps make it easier for students to separate the ice layers), and then place in freezer for a day.
- Make sure the first layer is completely frozen before adding the next layer or layers will mix and students will get confused. Be sure to add the correct data to the correct layer.
Introducing the Lab (30-60min discussion)
PRE-SURVEY
Please have each student fill out the Pre-Lab Survey (found at the end of the worksheet document). The point of this introduction is to expose students to different timescales – they should be able to explain the difference between tectonic/geologic, millennial to orbital, and anthropogenic timescales. Additionally, this introduction should answer any questions students may have about ice cores. Discuss the adventure and difficulties associated with obtaining a real ice core.
PRIOR KNOWLEDGE
Query the class to find out what they already know about past climate.
- How do scientists know about ancient CO2?
- How can we know what temperatures were like before thermometers were invented?
- When were thermometers invented ?
- When was the instrument that measures present day atmospheric CO2 levels invented? Who invented it?
- Why do we care about past climate?
TIMESCALES
Earth’s processes operate on a number of vastly different timescales, from diurnal, seasonal, decadal, anthropogenic, millennial, geologic/tectonic, and orbital. Understanding these timescales is one of the most important, fundamental concepts in paleoclimate. Before diving into the millennial to orbital timescale that this lab is devoted to, review changes in temperature and CO2 that occur on other timescales. Examining the Keeling Curve for a record of earth’s seasonal CO2 cycle is a good way to connect with what students already understand about photosynthesis and respiration. You could revisit the Keeling curve (https://scrippsco2.ucsd.edu/history_legacy/keeling_curve_lessons.html).
Have students visit the NOAA Paleoclimatology TimeLine http://www.ncdc.noaa.gov/paleo/ctl/index.html. Check out Earth maps from different periods in Earth history at https://deeptimemaps.com/. Chapter 12 (p.233) in Kump et al. is devoted to long-term climate variability, which is controlled by very different processes (for example silicate weathering in Chapter 8, p.159) than the climatic variations that occur on millennial or orbital timescales. Students may be surprised to learn that Earth is presently in an “Ice Age” (albeit an interglacial within the ice age) because there are large permanent ice sheets in both Polar Regions. Other Ice Ages are rare and occurred millions of years ago (geologic timescale). We can’t use ice cores to study ancient ice ages because that ice is long gone so the long-term climate record is inferred from deep-sea sediment cores (until about 200 million years ago), continental rocks, and geologic deposits (pp. 240 – 252 Kump et al.).
FIELDWORK
A good way to introduce the lab is to engage the students in an animated discussion about going to the field to obtain ice cores and then having them guess what the data will show. Some questions you might ask: It is time to plan an expedition to Antarctica! What does it take to plan a scientific field expedition? Who pays for it? Who is in charge of the logistics? What tools do we need? What clothes do we need? How do we get ice cores back to the lab? Where do we store them once they are here? How are we going to divvy up the ice to measure all the different parameters (CO2, N2O, CH4, stable isotopes, snow accumulation, grain radius, dust, conductivity, ionic chemistry…)? What do you think CO2 levels looked like in the past? Has temperature been constant during the last 20,000 years? The last 800,000 years? How do you think it has varied? Why has it varied?
Watch interviews with PCC climate scientists https://www.youtube.com/watch?v=jul5Tyzrg4c and a conversation with Spruce Schoenemann about his research on Antarctic ice cores https://www.youtube.com/watch?v=ywYfW0jps-w.
Part 1: What is the temperature proxy? (20-40 min discussion + activity)
In Part 1, Students learn how hydrogen isotopes are used as a proxy for temperature – and how marbles are a proxy for isotopes!
DISCUSSION/LESSON
A proxy is a substitute for an actual measurement. CO2 data from ice cores is not proxy data because the CO2 record is derived from measurements of CO2 in ancient air bubbles trapped in ice. However, ice core temperature data is derived from a proxy. Isotope measurements of the water molecules that make up the ice reveal past temperature changes. Isotopes are identical atoms with different masses due to different numbers of neutrons (see p. 15 and p. 274 in Kump et al. for the basics). There are two stable hydrogen isotopes: “protium” which is normal hydrogen with one electron, one proton, and zero neutrons, and “deuterium” which is the rare stable hydrogen isotope with one electron, one proton, and one neutron.
To start with glaciers have less of the heavy hydrogen isotope than does seawater. Glaciers are formed from water that evaporated from the oceans. When water evaporates the heavy water is left behind and the water vapor is enriched in light water. So the water that forms the ice in the glaciers has more of the heavy isotope than does the surface ocean. This “fractionation” occurs because it takes more energy to evaporate water molecules containing the heavy isotope from the surface oceans. Also, as the moist air is transported polewards and cools, the water molecules containing the heavier isotopes are preferentially rained out. The extent to which this “fractionation” occurs–during precipitation and evaporation–is dependent on temperature.
During a cold glacial period, the equator to pole temperature gradient is large and very few heavy hydrogen isotopes make it to the poles. They rain out very soon after leaving the ocean surface. During a warm interglacial however, there is more energy in the Earth system and the equator to pole temperature gradient is relatively small which leads to more water molecules with a heavy isotope reaching the pole.
ISOTOPE DATA NOTATION
Because deuterium is very rare, hydrogen isotope ratios are very small numbers and they need to be related to a standard. Thus isotope data is expressed in delta (δ) notation (the relative deviation of a sample’s isotopic ratio compared to the isotopic ratio of the standard VSMOW) with units in per mil (‰). Ice core “delta” values are negative because the D/H ratio of the ice core sample is compared to VSMOW. The “delta” value of mean ocean water (VSMOW) is zero. The ocean has more deuterium than most natural substances – so most samples have a negative value according to “delta” notation because they are depleted in deuterium relative to the standard. The units are per mil (‰) so that the numbers are not much less than unity.
Just knowing what the variations in isotopes are down an ice core can tell us a lot about changes in temperature – and that is as far as this lab goes. But this data can be taken a step further and be converted into actual temperature values. Spatial observations of isotopic variation in precipitation have allowed scientists to calibrate the relationship between temperature and isotopic ratios.
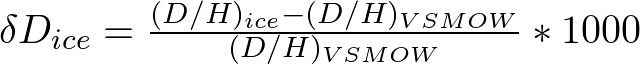
PROXY ACTIVITY
Key Point: Very negative δDice values represent colder temperatures and less negative values represent warmer climate conditions.
To illustrate this key point, try the following exercise
- Talk about isotopes and delta notation.
- Give each group the PROXY Worksheet found in the worksheets file.
- Point out that marbles are used here as proxies for isotopes–and isotopes are proxies for temperature!
- Provide groups of students with one of two sets of marbles Set 1: 3 big green marbles, 9 small purple marbles, and 7 small orange marbles. Set 2: 4 big green marbles, 4 small purple marbles, and 2 small orange marbles.
- Students use the worksheet to determine the isotopic ratio represented by their set of marbles (either -397‰ or -442‰).
- Write the findings on the board. To illustrate that students understand the main point of this exercise, ask them which δDice value represents a colder temperature? (Answer = the more negative -442‰). This crucial activity will ensure that each group understands how they are going to get isotope data from their ice core.
- Make sure everyone understands what all parts of the proxy worksheet mean (why the units for CO2 are PPMV and any other isotope clarifications).
Part 2: Dissecting the Ice Cores (30-45 min activity)
In this part, students explore a mock ice core, in a way that models how a scientist would gather data and analyze these samples. Students will learn how ice cores can reveal past climate and in what ways the climate during the Last Glacial Maximum was different than it is today.
DATING THE ICE
Dating ice cores is not a focus of this lab, but it should be mentioned how we determine the age of the gas and the age of the ice.
Layers of ice (if visible) are counted to infer the age of the ice core matching parameters to other known dates from proxies, tying ash layers to known volcanic eruptions, and using flow models to account for the movement of deep ice away from its original deposition location. Ways to improve age dating of the gas and the a ice is a very active area of research.
DISSECTING THE ICE CORES
Now that students are familiar with the special isotope proxy and entering their data on a worksheet – it is time to produce data! Distribute worksheets and review the parts that need to be filled out and what the units mean. Hand out ice cores, towels, hammer and chisel, hacksaw, cutting board, and cups. Everyone should wear safety goggles.
Allow 30-45 minutes for the dissection to proceed. Help students check that they don’t mix up CO2 data and marbles between layers. Everyone should enter the CO2 and temperature data extracted from the ice core on their own worksheet. Once a group has all the data from the four layers, help them enter it in the Excel spreadsheet (the tab called “Dissecting Ice cores”). The correct data is also graphed on the Excel tab called “Lab Answer Key” so you can easily check if a group mixed up any data. Once the graph is finished display the completed graph.
Explore some key points. If any student-generated points are different from the lab answer key, discuss the possibility of human error. What is going on during the transition from the Last Glacial Maximum to the present? Were CO2 concentrations higher or lower 20,000 years ago? What do the isotopes tell us – was it warmer or colder 20,000 years ago? Do the data meet our expectations? Are there any surprises? Is there anything strange about the graph? What are some possible reasons for the jumpy shape of the CO2 and Isotope plots? What does this data tell us about what has been happening to Earth’s climate since the Last Glacial Maximum? What kind of timescale does this data represent?
Part 3: Extending the Record (15-30 min discussion or homework)
The point of this activity is for students to be able to explain the character of glacial-interglacial cycles during the last 800,000 years and visualize the orbital timescale. Now that students have created and analyzed the 20,000-year CO2 record, let them extend that record another 740,000 years!
The Excel tab called “Extending the Record” has 1095 CO2 data points and 5787 temperature data points. You can make this a homework assignment by deleting the graph and asking students to create it. Ask discussion questions like “What triggered the CO2 and temperature to go up and down?” “Why are the glacial periods longer than interglacial periods?”
Here you can address a popular climate skeptic argument that some of your students may be familiar with, as well as starting to address local vs global phenomenon. Close inspection of the CO2 and isotope record from Dome C show a rise in temperature during the last transition from glacial to present that precedes an increase in atmospheric CO2. Skeptics latch onto this phenomenon and claim that CO2 does not cause temperatures to rise. However, this ice core record reflects local conditions at Dome C in Antarctica. Combining proxy records from around the world (Shakun et al. 2012) reveals that on a global scale CO2 increases before global temperatures, while local conditions in Antarctica stray from the global mean. Potential reasons for the local differences relate to the timing of changes in ocean circulation, sea ice cover, permafrost and terrestrial plant growth, and albedo – all of which were experiencing major changes during the transition. Regardless of the mechanism, the main point is that the timing of CO2 and temperature records from the Antarctic ice core represent local, not global, conditions and can therefore not be used as evidence to suggest that CO2 is not a cause for changes in temperatures. Also – it is possible that ice core ages and gas ages are not perfectly reconstructed.
Part 4: Adding Modern Data to the Record (30-60 min discussion or homework)
In this activity students learn to use scientific data to explain two key differences between anthropogenic CO2 and natural CO2 variability: rate and magnitude. They construct an argument about how natural variability is different than anthropogenic climate change.
The Excel tab called “Add Instrumental Data” includes two new data sets:
- the modern record from CO2 measurements made for the past 53 years, and
- a different ice core from Law Dome to connect the gap between the instrumental record and the Dome C ice core
There are two key ideas to focus on.
1. Observe the scale of human-caused CO2 change. Natural CO2 variability during the past 800,000 years has been between 180 and 300 ppmv. Looking at the instrumental record on these graphs you see that humans have added over 90 ppm to the atmosphere (since this lab was written note CO2 concentrations in May 2020 reached 415 ppmv, increasing this difference between natural and human-caused to 115 ppm).
2. This leads us to examine how fast CO2 changed in the past and how fast we are adding CO2 to the atmosphere now. It may help to change the scale on the x-axis or plot the best fit lines that describe CO2 increase at glacial-interglacial transitions and compare those slopes to the recent increase in atmospheric CO2. It took humans only 100 years to cause this change while it takes the natural cycle 10000 years to add 100 ppmv CO2 to the atmosphere.
Understanding how fast humans are altering the carbon cycle by burning fossil fuels and increasing atmospheric CO2 is one very important reason to study Earth’s past climate. Without this knowledge, we would have no baseline to compare the human impact on climate. Students should watch this instructive video on past CO2 fluctuations more than once! http://www.esrl.noaa.gov/gmd/ccgg/trends/history.html
References
Textbook: Kump LR, Kasting JF, Crane GC (2010) The Earth System. Pearson
Shakun, JD, Clark PU, He F, Marcott SA, Mix AC, Liu Z, Otto-Bliesner B, Schmittner A, Bard E (2012) Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation. Nature 484 pp. 49-56 (http://people.oregonstate.edu/~schmita2/pdf/S/shakun12nat.pdf)
Additional Resources
ICE CORES
- Similar data analysis activities based on the 400,000 year Vostok, Antarctica record
- Another approach to making ice cores that study weather events during a winter season from NASA’s Education Student Observation Network at: http://www.nasa.gov/audience/foreducators/son/winter/snow_ice/students/F_Snow_and_Ice_Students.html
PALEOCLIMATE
- Timescales: NOAA timeline http://www.ncdc.noaa.gov/paleo/ctl/index.html
- Paleoclimate animations http://emvc.geol.ucsb.edu/1_DownloadPage/Download_Page.html
- Paleoclimatology overview http://serc.carleton.edu/microbelife/topics/proxies/paleoclimate.html
- NOAA Paleoclimatology data portal http://www.ncdc.noaa.gov/paleo/paleo.html
- What’s the connection between low sea level and big ice sheets? Deep Sea Paleoclimate http://oceanexplorer.noaa.gov/explorations/05stepstones/background/paleoclimate/paleoclimate.html
- Antarctic marine sediment core lesson plan: http://serc.carleton.edu/eet/cores/index.html
MILANKOVITCH CYCLES
- Lesson: http://www.sciencecourseware.org/eec/GlobalWarming/Tutorials/Milankovitch/
- Visualization: https://cimss.ssec.wisc.edu/wxfest/Milankovitch/earthorbit.html

