4 Scattering Rays
So at the top of the Earth’s atmosphere, direct shortwave radiation shines with a strength of 1362 W/m2. A total of 173 PW absorbed on Earth’s surface, and averaged over the planet it amounts to 340 W/m2. What happens when all that radiation hits the air?
From one perspective, the answer is “not much.” The atmosphere is largely made of gases that don’t interact strongly with shortwave radiation. Nitrogen, for instance, makes up 78% of air and doesn’t interact strongly with sunlight, with the exception of Rayleigh scattering, the process that makes the sky blue. All in all, nearly 50% of incident shortwave radiation (163 W/m2, or 83 PW) makes it all the way to the Earth’s surface and is absorbed there. Another 30% is reflected back to space (100 W/m2, or 51 PW), while 23% is absorbed within the atmosphere (77 W/m2, or 39 PW).
What does the reflection? The critical concept here is albedo, the reflectiveness of a surface. White objects like snow or clouds have high albedo, because they reflect back most of the incident visible light. Darker objects like the ocean or forests have low albedo.
| Substance | Albedo |
| Clouds (depends on thickness) | 0.2-0.8 |
| Snow (depends primarily on age) | 0.4-0.9 |
| Desert | ~0.3 |
| Ocean | < 0.1 |
Clouds reflect the most light in the global average. There are a few different ways to attribute the amount, since sometimes there are multiple high albedo surfaces in a single column. If you consider the method that attributes the least of the reflection to clouds, that is, the difference in shortwave radiation between cloudy and cloud-free conditions, clouds cause 47 W/m2 (47%, or 24 PW) of the reflected radiation. This quantity is called the shortwave cloud radiative effect (SWCRE). SWCRE attributes less reflection to clouds because even thick clouds over a hypothetical perfectly reflective surface would have no cloud radiative effect. Other methods attribute to clouds about two-thirds of the total reflection.

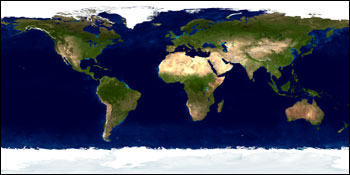
The Earth with clouds and no clouds, NASA.
Although surface albedo plays a critical role in determining local climate in places like the Arctic, it is less important than clouds in determining the average climate of Earth. Only 23 W/m2 are reflected back to space by the surface (it’s been estimated that this number would be 33 W/m2 if there were no clouds). There is 17 W/m2 reflected back by the non-cloudy atmosphere, and this would be about 20 W/m2 without clouds.
The substances that are most important for the clear sky reflection are aerosols, discussed in our “everything off” thought experiment in Chapter 1. There are plenty of important natural aerosols too: sea salt and dust are two prominent examples. Dust can be anthropogenic too, near agricultural operations that strip topsoil, or near deserts.Global emissions of sulfur dioxide by sector. Data from CEDS.
Because sulfate particles are reflective, they cool the planet. They also help clouds to form, which also reflect away sunlight. Both of these effects, known as the direct and indirect effect of aerosols, respectively, are important in causing cooling from pollution.
Global emissions of black carbon by sector. Data from CEDS.
Some aerosols are absorbing, particularly soot (also known as black carbon). Included in the cooling figure above is an offsetting heating from absorption of sunlight by soot in the atmosphere, and after it falls on the ground into snow.
Radiative forcing is a measure of how a given change in some aspect of the climate (either industrial-caused or natural) has affected the Earth’s energy budget. For quantities that affect shortwave radiation, radiative forcing is calculated simply as the change in shortwave radiation absorbed on Earth. Positive radiative forcing indicates more absorbed energy, and therefore cause warming. The best estimate of radiative forcing from aerosols and its uncertainty is plotted below.
Radiative forcing from particulate matter since 1850, including the envelope of scientific uncertainty, in W/m2 (inputs4MIP and IPCC).
Cooling from particulate matter doubled between 1950 and 1980. Since that time, the pollution-induced cooling has remained steady, although different regions of the world have increased or decreased their pollution much more than the global average. Clean Air Acts in the United States and Europe have been successful at reducing the asthma-inducing aerosol pollution in many, but not all communities. As the US and Europe reduced its pollution, countries like China and India saw pollution skyrocket, largely due to increased burning of coal for electricity and industry.
The uncertainty in particulate matter forcing is huge, over 1.2 W/m2 right now. We just aren’t sure how much effect the pollution is having on sunlight. This is in part because we don’t have a clean preindustrial atmosphere for comparison. The role of haze is one of the most important uncertainties in the projection of future climate.
No air
What would the Earth’s temperature be if there was no atmosphere? Let’s start with the assumption that the observed amount of shortwave radiation is absorbed, that is
[latex]S_{net} = S_{down} - S_{up}\\ = 340 W/m^2 - 100 W/m^2 \\ = 240 W/m^2[/latex]
with Snet = net shortwave radiation absorbed, Sdown = downward shortwave at top of atmosphere, and Sup = upward (reflected) shortwave at top of atmosphere.
If we assume that surface emits like a black body, and the planet is in energy balance, the global-averaged energy balance equation for our airless planet is
[latex]S_{net} = \sigma T_s^4[/latex]
where is the σ = the Stefan-Boltzmann constant = 5.67e-8 W/m2/K4 and Ts is the global-averaged surface temperature.
Solving for Ts, we obtain the very cold temperature of 255 K = -18o C = 0o F. This is about 33o C colder than the observed global averaged temperature of 15o C. The Earth would be a frigid place indeed if it wasn’t for the greenhouse effect.
The reflectiveness of a surface to visible light. An albedo of 1 corresponds to perfect reflection, while 0 means all light is absorbed.
Tiny water droplets or liquid water crystals suspended in the air. Don't confuse clouds with water vapor, the gas phase of water, which is invisible.
Aerosols are mixtures of tiny liquid or solid particles suspended in air. Aerosols can either scatter or absorb sunlight, and currently aerosols have a negative radiative forcing on climate.

