Introduction to Cell, Tissue Ultrastructure
SESSION OBJECTIVES:
Use these session objectives to test your knowledge of the important concepts presented in this chapter and as study topics to return to prior to your exams. These pre-class materials and virtual lab exercises are designed to help you achieve each objective.
- Describe the preparation of tissues for study including fixation, embedding and sectioning, and staining.
- Compare the appearance of different basic histologic stains.
- Present comprehensive definitions, descriptions and knowledge of locations of the structural components of eukaryotic cells.
- Demonstrate an ability to recognize the general characteristics and specific features of normal cells as viewed by light and electron microscopy.
- Develop the ability to interpret microscopic images and to relate these two-dimensional images to the three-dimensional features of tissues and organs.
OPTIONAL PRE-CLASS MATERIALS FOR THIS SESSION:
- Skim the section titles, bolded terms, and image captions from Junqueira’s Basic Histology, Chapters 1-3 (Click here for FMR library resources) to fill in any knowledge gaps you feel you have.
OVERVIEW:
This is a brief overview of the cellular ultrastructure and the cytoskeleton. Use the following activities and links to practice identifying key cellular structures commonly viewed via light microscopy using standard staining techniques and transmission electron micrographs. Each section is arranged by session objective.
Optional Activity: Click for an overview of the cell: HISTOLOGY GUIDE VIRTUAL MICROSCOPE
There are a variety of links to the Histology Guide Virtual Microscope to practice finding the cells that are discussed in your pre-class and class materials. These activities will help you recognize specific staining properties, cell organelles, cell types, and characteristics of body tissues. These are optional activities, but if you are struggling with identifying histological preparations and cells compared to your pathology sections, the histopathology thread instructors suggest that you practice using these links to help you study.
TISSUE PROCESSING (Session Objective #1)
BASIC CELL STRUCTURE (Session Objective #3)
Knowledge check: Identify each cellular structure and then click the question marks to check your answer:
Knowledge check:
HISTOLOGICAL STAINS (Session Objective #2)
Now, answer this basic concept question:
Can you identify why the section stains in this manner?
Now let’s look at some micrographs!
Hematoxylin and Eosin Stain (Session Objectives #2 and #5)
H&E Stain Color Key:
- Nuclei usually stain dark blue
- Cytoplasm of cells a variable pink to purple depending on the structures in the cytoplasm and cellular processes. In the image above, the cytoplasm is staining with eosin.
- Proteins some shade of pink/red
This is probably the most widely used stain combination.
In the drawing of the large intestine below on the left, you can see the three dimensional aspect of the structure of the gut tube as having a lumen (L) of the tube itself and a wall (W) enclosing the the lumen of the organ. In the section of large intestine on the right, the luminal epithelium (E) is toward the bottom of the micrograph, and the smooth muscle of the gut wall is toward the top of the image(SM).

At higher magnification, the micrograph of the same section of large intestine below shows the three major layers of the large colon: The purplish epithelial layers (E) that are continuous with the lumen of the gut tube, the outer layer of smooth muscle (SM) making up the wall of the intestine, and a middle connective tissue layer (CT). The different layers can be distinguished by the organization and density of cells/number of nuclei.
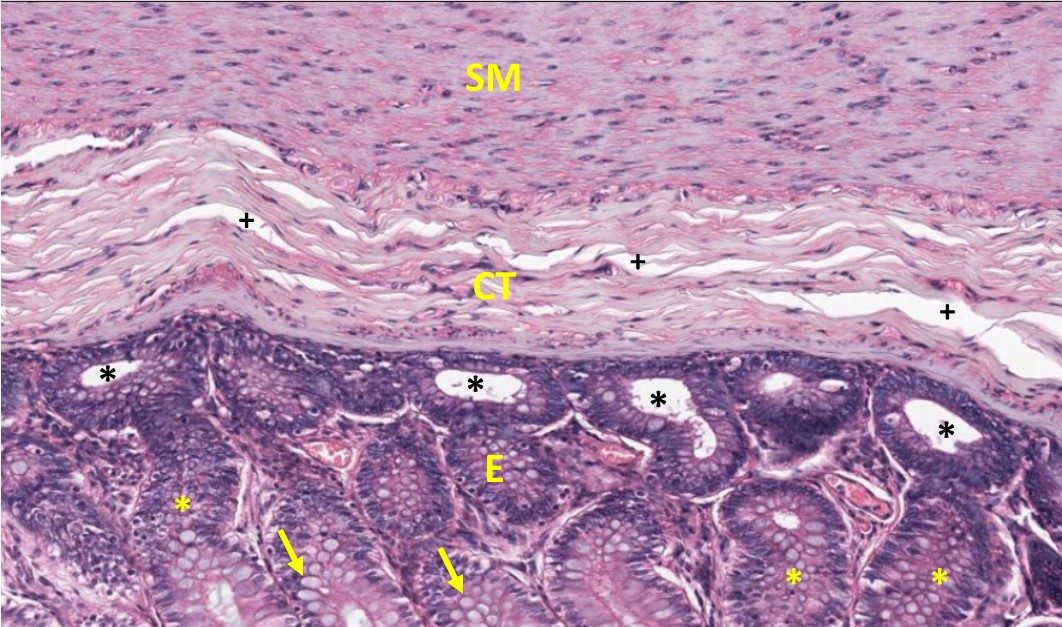
Ranking nuclear density (#nuclei/area) based on this image:
E(epithelium) has greater (>) nuclear density than SM (smooth muscle) which is > CT (connective tissue).
The density of the epithelial nuclei make this a “basophilic” compartment of the tissue. The epithelial cells are arranged as invaginated tubes that open onto the gut lumen, and in the section above, some of these tubes are cut obliquely (yellow asterisks) and others are cut in cross section (black asterisks). Also note the scattered epithelial cells that are not well stained and appear like little bubbles (yellow arrows). These cells are called goblet cells and they produce mucus which does not stain well with H&E. This mucus aids in the movement of fecal contents through the large intestine. This sample also introduces you to a common artifact of histological samples-‐shrinkage-‐which is most prominent in the CT layer (plus signs), where the white spaces caused by tissue processing generates spaces that do not occur naturally.
Optional activity: Additional practice opportunities and description of H&E staining.
Comparing H&E to other stains (Session Objective #2):
- PAS: If we use an H&E stained section of the large intestine and then counter stain it with another stain called Periodic Acid Shiff (PAS), we can now view the goblet cells (yellow arrows) better. The PAS stain is used to highlight carbohydrate moieties. In this case the mucus produced by epithelial cells as well as the mucus that is secreted into the gut lumen appears magenta.
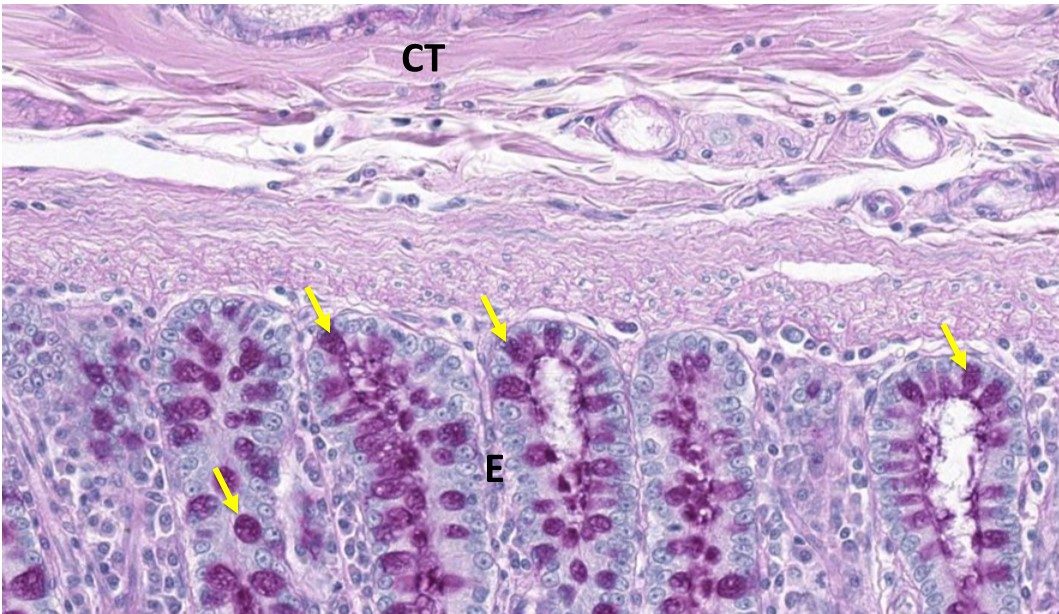
Optional activity: Additional information on PAS stain with cellular descriptions.
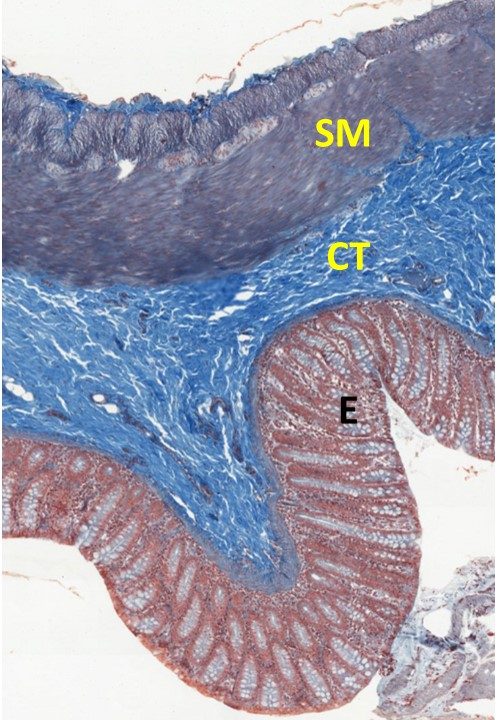
- Mallory or Trichrome Stain: Again we will examine a section of the large intestine. This section has been rotated slightly so that the lumen is on the lower left of the micrograph. The layers are the same as the sections we have viewed in the last two sections.
Trichrome Stain Color Key:
-
- Nuclei stain red-purple
- Connective tissue elements stain bright or light blue
- Cytoplasm and cytoplasmic proteins are a range of red – purple colors.
- Toluidine Blue: This is another basic dye, similar to hemotoxylin, and so it binds cellular structures that have a negative charge. The image below is a similar section of large intestine stained with toluidine blue.
Toluidine Blue Stain Color Key:
-
- Chromatin stains dark purple/blue
- Cytoplasm and connective tissue elements stain light blue/violet

-
- Optional activity: Additional sections stained with toluidine blue.
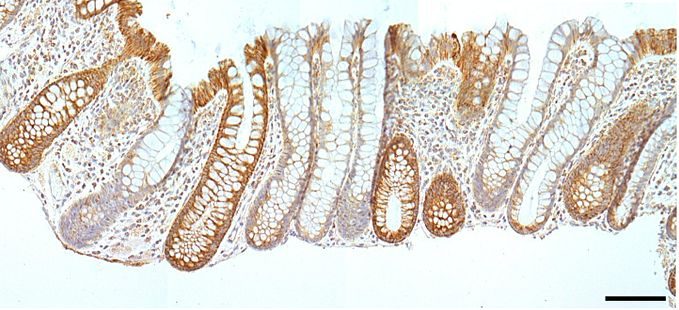
- Immunohistochemistry (IHC): IHC is a technique you will see in the histopathology thread as it is a way to visualize specific proteins of interest using primary and secondary antibodies. It will appear as a brown color on sections, like the hemotoxylin and IHC section of the large intestine shown below:
TRANSMISSION ELECTRON MICROSCOPY (Session Objective #4)
You can see cellular organelles in most detail using transmission electron microscopy. This technique uses a different fix and tissue processing method, as well as embedding the tissue in a resin rather than paraffin. The harder resin and tissue processing method allows for smaller and thinner sections to be sectioned to see finer detail.
Matching Exercise:
Optional activity: Want more?
- You can toggle between grayscale and color filled TEM micrographs to get more familiar with cellular organelles . Use the color option in the right panel of the screen to turn the color on/off and use the numbers at the bottom of the right panel to advance through the activity.
CELLULAR ACTIVITY (Session Objectives #3 and #4)
Answer the following questions about what cellular process is occurring based on the morphology of the cell(s) in the micrographs:
Optional activity: A description of the phases of mitosis. Click on the numbers in the right panel on the screen to view specific examples of mitotic figures in different phases of mitosis.
This Chapter’s PDF
HIST: Introduction to Cell, Tissue Ultrastructure
- Note: The interactive features of this chapter are not reproducible in this PDF format
ARCHIVAL MATERIALS:
- Pre-recorded videos: Introduction the Cellular Ultrastructure and the Cytoskeleton
- Part 1 (26:48)
- Error correction: On slide 11 in the video, the term microvilli (a cellular membrane specialization) was used in the video in place of the correct term villi (a fold of the wall of the small intestine) when referencing the oblique, transverse, and longitudinal sections through a section of duodenum.
- Part 2 (21:25)
- Video Powerpoint file
- Part 1 (26:48)

Feedback/Errata