Bone
SESSION OBJECTIVES
- Describe the structural features of bone and the relationship between the different kinds of cells and the mineralized and non-mineralized organic matrix.
- Describe how bone grows and is remodeled.
- Describe and contrast endochondral and intramembranous bone formation.
- List the hormones that control bone growth, and that regulate blood calcium levels.
- Understand the basis of bone disorders and the phases of bone repair after a fracture.
OPTIONAL PRE-CLASS MATERIALS FOR THIS SESSION:
- Skim the section titles, bolded terms, and image captions from Junqueira’s Basic Histology, Chapter 8 (Click here for FMR library resources) to fill in any knowledge gaps you feel you have.
GENERAL CHARACTERISTICS OF BONE TISSUE (Session Objective #1)
- Basic composition: Bone is a dense organic matrix of collagen fibers (Type I), containing sulfated proteoglycans. Recently formed, non-mineralized organic matrix is called osteoid. Hydroxyapatite (calcium phosphate) crystals are deposited on the collagen fibers and ground substance of the osteoid to form bone. Together, the mineral component and non-mineral matrix are responsible for giving bone both hardness and resistance to deformation (toughness).
Material property of bone: Hardness:
Material property of bone: Toughness (After you have answered the question, you don’t need to watch the rest of the movie):
With both a tough collagen scaffolding and a hard mineral shell, you have an amazingly resilient, self-repairing structure!
- Surface linings and bone cells: The Periosteum and endosteum are the outer and thin inner connective tissue coverings of bone, respectively. They contain the stem cells for osteoblasts and carry the blood vessels nurturing the bone. Small branches from periosteal and endosteal vessels penetrate bone via Volkmann’s canals. The periosteum is attached to bone by collagen bundles that penetrate into the bone (periosteal fibers or Sharpey’s fibers). The periosteum has two layers, an outer fibrous layer and an inner cellular and vascular layer.
A highly dynamic structure, bone is constantly being maintained. Four main cells are associated with regulating bone maintenance:
Knowledge check: Mentally identify each bone cell on the image below before revealing the answer:
-
- Osteoprogenitor cells:
- Location: Periosteum and endosteum
- Structure: Small elongated cells.
- Function: Serve as the stem cells of bone by differentiating into osteoblasts, referred to as osteogenic cells.
- Osteoblast cells:
- Location: Lining the surfaces of bones, particularly contributing to the bulk of the endosteal layer.
- Structure: Differentiated osteoprogenitor cells that are cuboidal in shape.
- Function: These cells function to build bone. Osteoblasts form the osteoid for hydroxyappatite crystals to form on and harden the organic matrix.
- Osteocytes:
- Location: Trapped in lacunae within bone tissue.
- Structure: Differentiated osteoblasts that have a star-shape resulting from cytoplasmic processes extending through small, bony canals called canaliculi to communicate with neighboring osteocytes. In standard paraffin histological preparations, the osteocytes often appear to be crumpled within or absent from their lacunae.
- Function: Maintain the calcified matrix by receiving nutrients and mechanical signals from their surroundings.
4. Osteoclast cells:
- Location: On the surfaces of bone.
- Structure: Giant, multinucleated cells derived from monocytes. Often have a “foamy” cytoplasmic appearance reflecting the transcellular transport of protons, organic matrix fragments, and minerals to and from the surface of the bone being degraded. The cell membrane surface of the osteoclast that attaches to the bone matrix takes on a “ruffled border” that also reflects the degradation processes occurring between cell and calcified matrix. The bone surface becomes scalloped in a characteristic pattern where bone is being digested under the osteoclast, called “Howship’s lacunae“, forming a resorption pit.
- Function: Digestion and removal of calcified bone matrix.
- Osteoprogenitor cells:
- Functions of bone:
- Support
- Protection
- Movement
- Cavities (red marrow) are the residence for hematopoietic stem cells and hematopoeisis
- Reservoir of calcium, phosphate, and other ions required for homeostasis
- Additional Information about Movement: Articulation of bones at freely movable joints are called diarthroses. This type of joint is also called a synovial joint, where the articulating bones are not directly attached to each other. Instead, the articulating ends of the bones are covered with hyaline cartilage (also called articular cartilage) and these articular surfaces are enclosed within a membrane-delimited space. The membrane that forms the synovial joint space between the two bones is the synovial membrane. This membrane participates in the formation of a thick viscous fluid that fills the space between the articular surface to lubricate and nourish them, called synovial fluid. While the synovial membrane is critical for the production of synovial fluid, it is not very strong mechanically and is reinforced with a connective tissue capsule that is comprised of sheets and bands of dense irregular connective tissue. Some synovial joints also contain additional bits of cartilage that improve the congruence (fit) between articulating bones (menisci in the knee joint, for example).
BONE STRUCTURE (Session Objective #1)
Knowledge check: Mentally identify the features of a typical long bone before clicking to reveal the answers (enlarge the image so it fits on your screen):
The shaft of a long bone (diaphysis) is composed of compact bone and contains the yellow marrow, while the ends (epiphyses) are formed mostly of spongy or cancellous bone and contains the red marrow. The junction between the epiphyses and diaphyses forms a “neck” called the metaphyses. Flat bones of the cranium have two plates of compact bone separated by spongy bone (diploe).
- PRIMARY BONE
Primary bone (woven, immature, nonlamellar) has randomly organized collagen fibers. It is temporary and replaced by secondary bone through remodeling. Primary bone has a lower mineral content and more osteocytes than secondary and in adults is found in areas of bone remodeling, tooth sockets, and the insertion sites of some tendons.
- SECONDARY BONE
Secondary bone (lamellar, mature) has collagen fibers arranged in alternating layers (lamellae), spiraling in opposite directions. Osteocytes usually sit in lacunae between adjacent lamellae. The lamellae surround canals containing blood vessels, nerves, and loose connective tissue. The canals are called Haversian canals, and a canal plus its associated lamellae is a Haversian system or osteon. Haversian systems receive blood vessels from the periosteum (or endosteum) via Volkmann’s canals. In sections, Haversian canals can be distinguished from Volkmann’s canals by their lamellae and because they run longitudinally in the diaphysis. Volkmann’s canals run transversely or obliquely and lack surrounding lamellae.
- LAMELLAE
Lamellae are layers of mature bone, much like rings of a tree. They are organized in three ways:
- Concentric lamellae of Haversian systems as described above; like rings of a tree.
- Circumferential lamellae at the external and internal surfaces of bone (internal to the periosteum and endosteum). The outer circumferential lamellae are easy to see, and run around the outer circumference of the bone. These are not parts of osteons.
- Interstitial lamellae lie between Haversian systems and appear in sections as triangular or irregularly shaped areas. They are parts of old Haversian systems that remain after bone remodeling. They still contain living osteocytes, but without a Haversian system supplying blood, they will be eventually replaced.
BONE FORMATION (Session Objective #3)
1. INTRAMEMBRANOUS OSSIFICATION
Intramembranous ossification is the simpler form of bone formation; it takes place within connective tissue membranes. It contributes to the growth of some short bones, to the thickening of long bones, and to the formation of cranial and facial bones. Stem cells in connective tissue give rise to osteoblasts in primary ossification centers and they in turn form bone matrix. Enclosed osteoblasts become osteocytes and new osteoblasts arise in surrounding connective tissue to continue the process of bone formation.
2. ENDOCHONDRAL OSSIFICATION
Endochondral ossification takes place on a hyaline cartilage model of bone. This is the method of bone formation in most long and short bones. It occurs by hypertrophy and death of chondrocytes accompanied by calcification of the cartilage matrix. This is followed by invasion of blood vessels and osteoblast precursors, development of osteoblasts, and the deposition of bone over calcified cartilage. These latter steps are part of the growth process of long bones (see histology atlas below for an indepth pictoral guide to the zones of endochondral ossification).
BONE GROWTH AND REMODELING (Session Objective #2)
1. LONG BONE GROWTH
The hyaline cartilage cells of the epiphyseal plates of long bones continue to divide and form bone by endochondral bone formation until maturity. Interstitial growth of cartilage is the method by which long bones grow in length. Areas of long bone growth are divided into five zones: resting, proliferative, hypertrophic cartilage, calcified cartilage, and ossification.
2. BONE REMODELING
Bones represent a dynamic source of calcium ions. As bone is deposited (formation), calcium is stored as insoluble hydroxyapatite. Normal degradation (resorption)of bone releases calcium ions into the circulation, and bone formation must again replace the bone that was degraded.
HORMONE CONTROL OF BONE GROWTH AND REMODELING (Session Objective #4)
GROWTH AND REMODELING
Cartilage and bone cells divide in response to Somatomedin C (Insulin-like Growth Factor) from the liver, which has been stimulated by Growth Hormone from the anterior pituitary.
Increasing levels of sex hormones (Androgens and Estrogens) inhibit and eventually stop this process. Once all the cartilage in the epiphyseal plate has been converted to bone, no more bone elongation is possible.
In Adulthood, the sex hormones play a significant role in bone homeostasis. Evidence of this is the loss of the protective effects estrogen has on bone in post-menopausal women who are more susceptible to developing osteoporosis.
Bone turnover and calcium homeostasis is regulated by the action of hormones produced by endocrine tissues. Parathyroid hormone increases bone resorption and therefore blood calcium levels, and calcitonin from the thyroid inhibits this process.

FRACTURE REPAIR AND REMODELING
Damage to bone results in the death of osteocytes. The bone matrix breaks down locally, and connective tissue producing cells invade from the periosteum and endosteum. Small patches of cartilage appear, endochondral and intramembranous ossification occur, and a bone callus of irregular trabeculae or primary bone surrounds, say, the fracture site. This bone callus is later remodeled into secondary bone. There are three phases of bone fracture and repair:
- Trauma: Blood clot formation/hematoma with fibroblast influx and revascularization. Appearance of chondrocytes that deposit collagen, which is later calcificed into a calcified matrix.
- Repair: Replacement of calcified cartilage with woven bone (4-6 weeks).
- Remodeling: Remodeling of woven bone to lamellar bone (6+ months).
BONE DISORDERS
- Already mentioned: Osteoporosis. This disease increases the rate of resorption over that bone formation which results in the loss of bone mass and strength. This condition is estrogen responsive. Lack of weight bearing exercise on the skeleton can also contribute to this condition called disuse osteoporosis. Here the osteocytes signal the breakdown of bone with the loss of mechanical force signals transmitted along their cellular processes.
- Osteopenia: Lower mineral density of bone, once classified as pre-osteoporosis
- Osteogenesis imperfecta: Recall that this is a genetic condition that results in the poorly formed and/or reduced production of functional collagen type I. The bones are exceptionally brittle (“glass bone disease”).
- Poor Diet
- Scurvy: Vitamin C deficiency impairs collagen and osteoid secretion.
- Rickets: Calcium/phosphate or Vitamin D deficiency in children. Results in poor transport by the gut epithelium which leads to poorly mineralized bone with a normal organic matrix. The result is more pliable bones and the characteristic bowed leg deformities (although it can occur in other locations besides the legs).
- Osteomalacia: Phosphate/Vitamin D deficiency in adults = decreased mineral content of bone and the accumulation of poorly mineralized osteoid.
- Osteopetrosis: There are fewer bone disorders that have excessive bone formation rates over those of bone resorption. Aside from some cancers, osteopetrosis is one of them. The osteoclasts have impaired function with this condition and so bone fails to remodel properly. With more mineralized bone (normal osteoblast bone deposition outpaces that of resorption) the skeleton is more brittle and prone to fracture. The marrow compartment where hematopoiesis takes places is also abnormal.
HISTOLOGY ATLAS AND PRACTICE EXERCISES
SLIDE 1. Bone: Compact and Trabecular (spongy) (H&E), Low Magnification
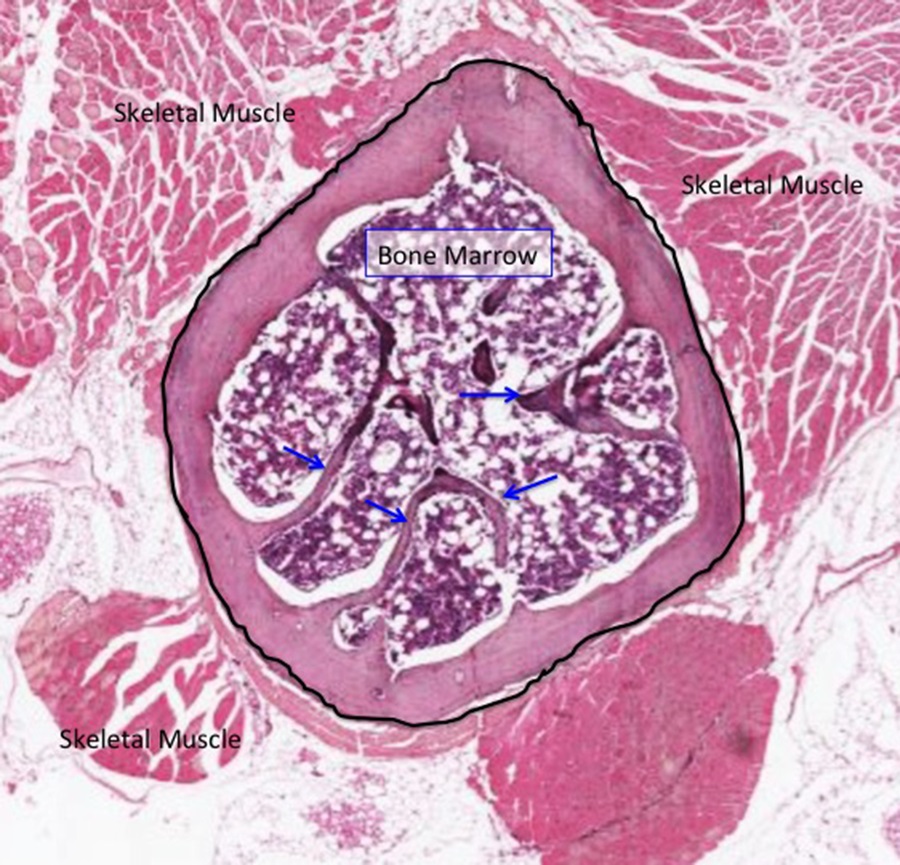
The light micrograph above shows a rib with hematopoietic bone marrow in its interior. Preparation of bone for histological imaging includes de-mineralizing the organic matrix in order to cut the bone in paraffin wax successfully. Even so, the marrow cells are damaged during sectioning on a microtome and tend to pull away from the surfaces of bone. Oftentimes, the cells inside the marrow cavities can clump, causing overlapping cells on the slide instead of remaining as a single layer. The osteocytes within the remaining organic matrix tend to appear withered inside their lacunae or are torn out altogether despite the fact that the collagen usually remains nicely intact. In the field above, note the outer cortical bone (circled) with projections of trabecular bone in the bone marrow (arrows). The cortical bone is not yet arranged in Haversian systems (osteons), but individual osteocytes can be identified at higher magnification in the image below. The endosteum has been pulled away from the bone of the marrow cavity during tissue processing.
Same Section, Higher Magnification
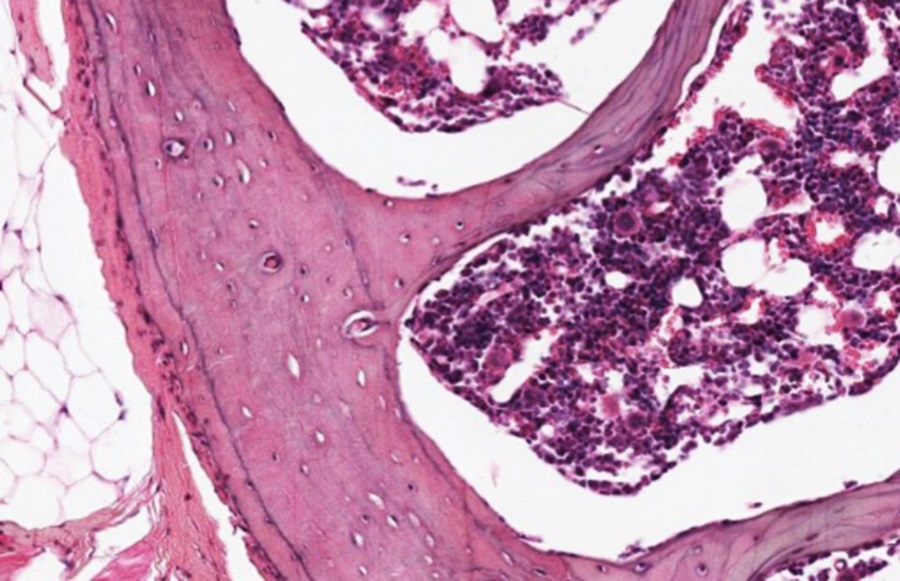
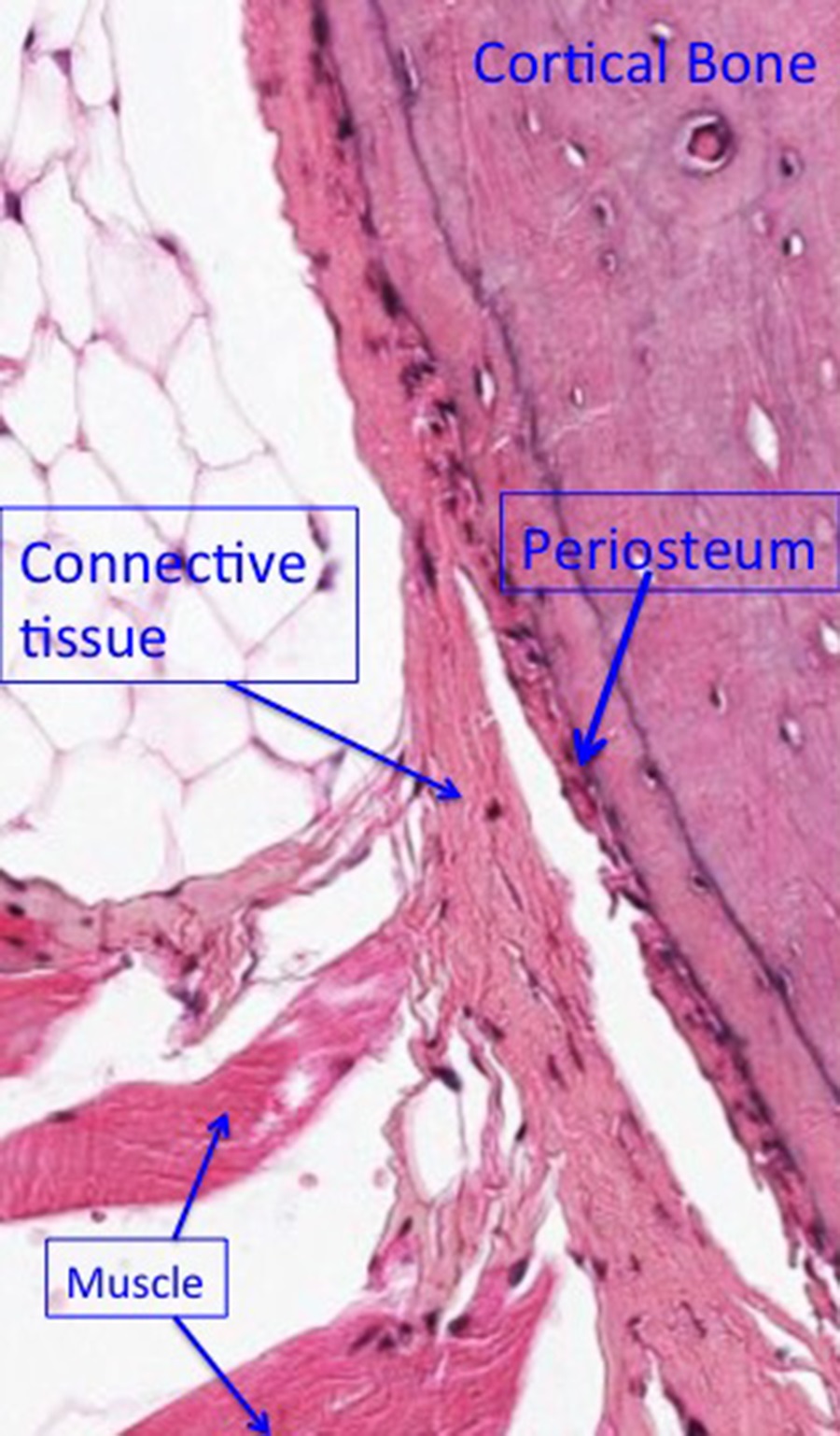
Around the circumference of the bone is a fairly dense layer of connective tissue-periosteum-with bone-forming potential. It blends into the dense irregular connective tissue that provides muscle attachment to bone (see below).
Optional activity:You can compare and contrast compact and cancelous bone at this link.
Optional activity:You can practice looking at a the structures of compact bone using a virtual microscope with guiding text in the right panel at this link.
Optional activity:You can practice looking at a the structures of trabecular/spongy bone using a virtual microscope with guiding text in the right panel at this link.
Slide 2. Ground Bone Section (Minerals remain intact), Low Magnification

The micrograph above is a piece of bone that was embedded in hard plastic and that is progressively ground down to yield a very thin specimen. Air and bone dust have filled the central canals (Haversian canals-yellow asterisks) and osteocyte lacunae to render them black. This specimen demonstrates the organization of osteons.
Yellow Box Above at Higher Magnification:

As bone is remodeled constantly, new osteons are replacing old ones by bone resorption and new bone deposition. Thus, newer osteons overlap older ones. This is demonstrated in the image above, where newer osteons (1; outlined in yellow) can be seen to partially obliterate older osteons (2; outlined in blue), which in turn have partially replaced other, older osteons (3, outlined in black). On the image above, the central canal of the osteons are indicated by asterisks and the canals that run at right angles to the osteons and connect them (Volkmann’s canals) are indicated by arrows.
Even Higher Magnification:
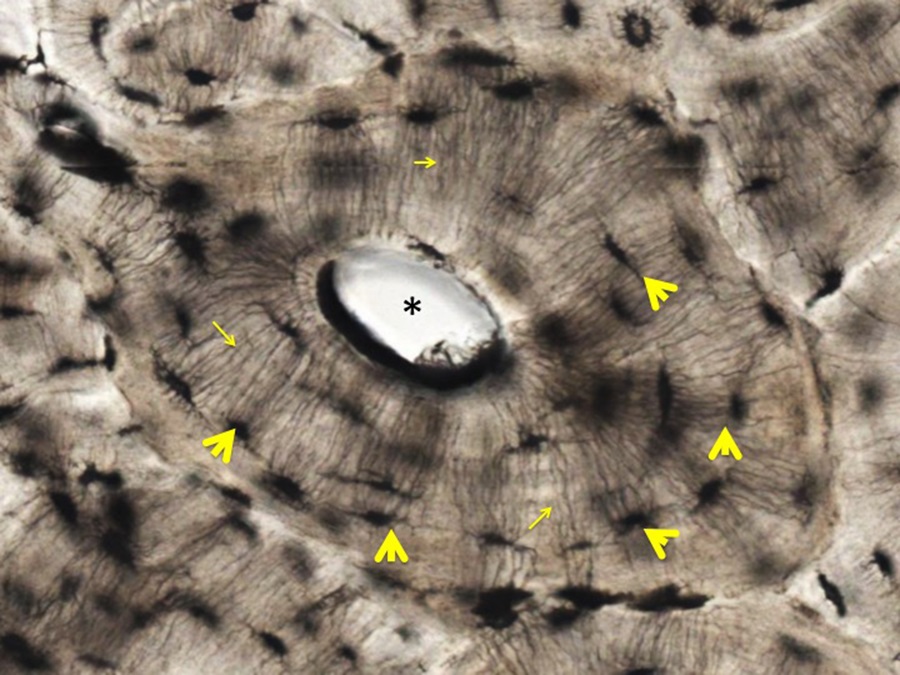
As shown in the panel above, the lacunae that once housed osteocytes (yellow arrowheads) and the very delicate canaliculi that connect the lacunae to each other and to the central canal (small yellow arrows) are evident. Regulation of bone resorption and deposition is a key mechanism to control levels of soluble calcium in the circulation.
Optional activity:You can practice looking at a osteons using a virtual microscope with guiding text in the right panel at this link.
Slide 3. Developing bone: Finger

Stain Key: Mallory-Trichrome Stain
- Nuclei and many proteins = varying shades of red
- Collagen of connective tissue and bone matrix = varying shades of blue
The image above shows the distal head of a metacarpal bone and parts of three phalanges.
Due to their relatively early stage of development, none of these bones have secondary ossification sites, only primary ossification sites in the region of the metaphyses. The epiphyses are indicated by asterisks and some bone matrix (blue with this stain) is outlined in yellow. The space destined to become the synovial space at the metacarpal-phalangeal joint is outlined in black at the metacarpophalangeal joint (image right).
Same Section, Higher Magnification

The osteoblasts, osteoclasts and osteoprogenitor cells collectively are a collection of cells known as the endosteum (enclosed by yellow squiggly line). The bone matrix is blue and contains many osteocytes. This immature bone has not yet organized into compact or lamellar bone and the osteocytes are scattered. The yellow box encloses a region of dense connective tissue on the outer surface of the developing bone. This is the periosteum that contains osteoblast progenitor cells.
Several developing osteons are can be seen at this magnification below. One is outlined in yellow. As blood vessels (yellow asterisks) penetrate the bone, they bring in osteoblasts with the accompanying connective tissue and osteoclasts from the blood itself.

Same Section, Even Higher Magnification
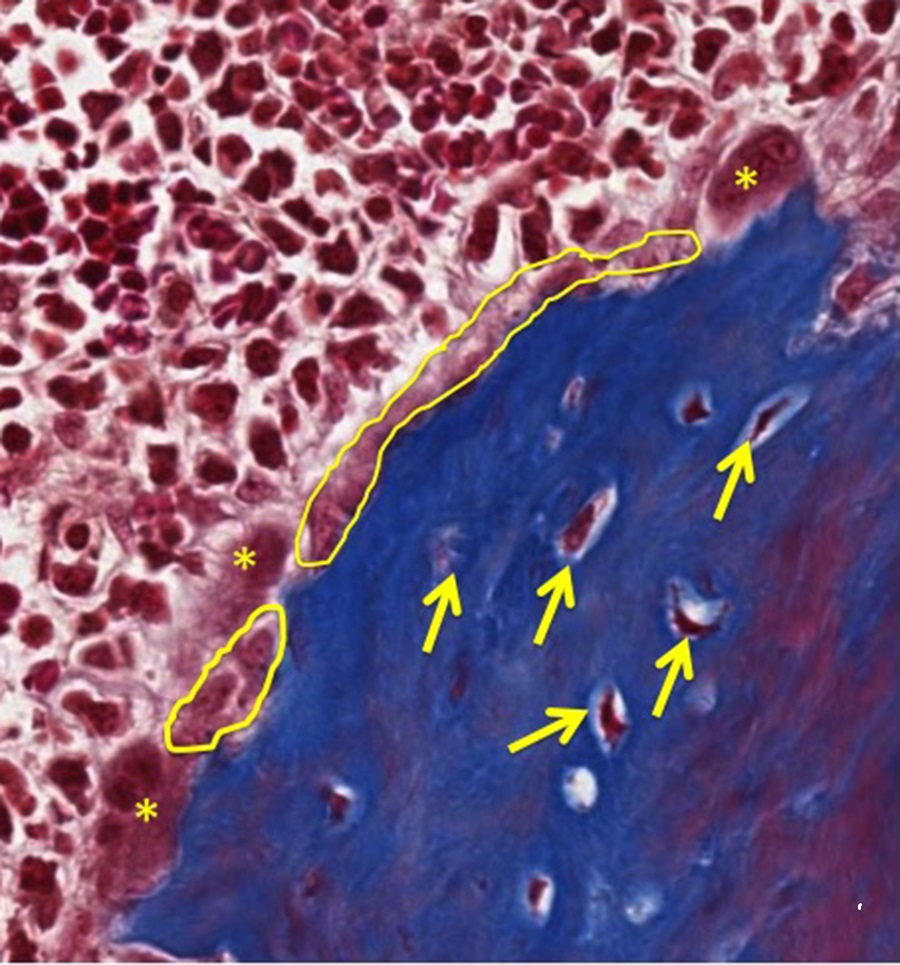
At higher magnification, the endosteum is highlighted in yellow and osteoclasts are indicated by asterisks. Randomly distributed osteocytes (indicated by yellow arrows) are embedded in bone matrix.
Slide 4. Developing bone- (Mallory-Trichrome)

Above is a developing femur (F), tibia (T) and patella (P). These developing bones are enveloped in immature skeletal muscle (M). This limb is more developed than the finger above-here secondary ossification sites in the epiphyses are evident (asterisks) and separated from the metaphysis by epiphyseal plates (indicated by black lines). The region indicated by the yellow box on the femur is a good site to examine the cellular constituents and organization of primary/woven bone that will become lamellar bone. The yellow box associated with tibial epiphyseal plate that separates the metaphysis from the epiphysis is a good place to study the cellular composition and process of endochondral bone formation.
Right Yellow Box From Micrograph Above: Higher Magnification of Femoral Primary Bone:
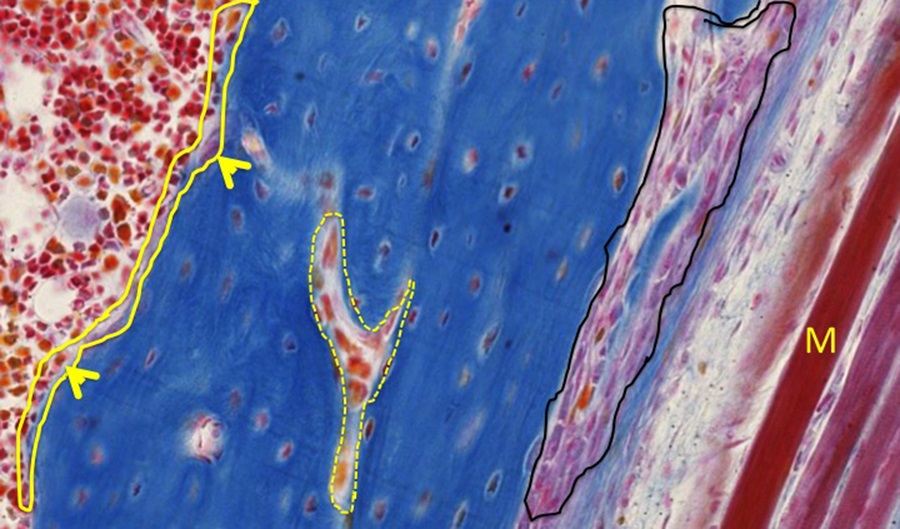
Along the femur, the endosteum location is outlined in yellow.The largest cells making up the endosteum are osteoclasts (arrowheads). A developing Haversian system/osteon is highlighted by a dashed yellow line. The periosteum is indicated in black. To the right of the periosteum are several immature muscle fibers (M) that will attach to the periosteal dense connective tissue. There are numerous osteocytes within lacunae of bone matrix. Lamellar bone has not yet formed.
Left Yellow Box From Micrograph Above: Higher Magnification of Epiphyseal Plate:
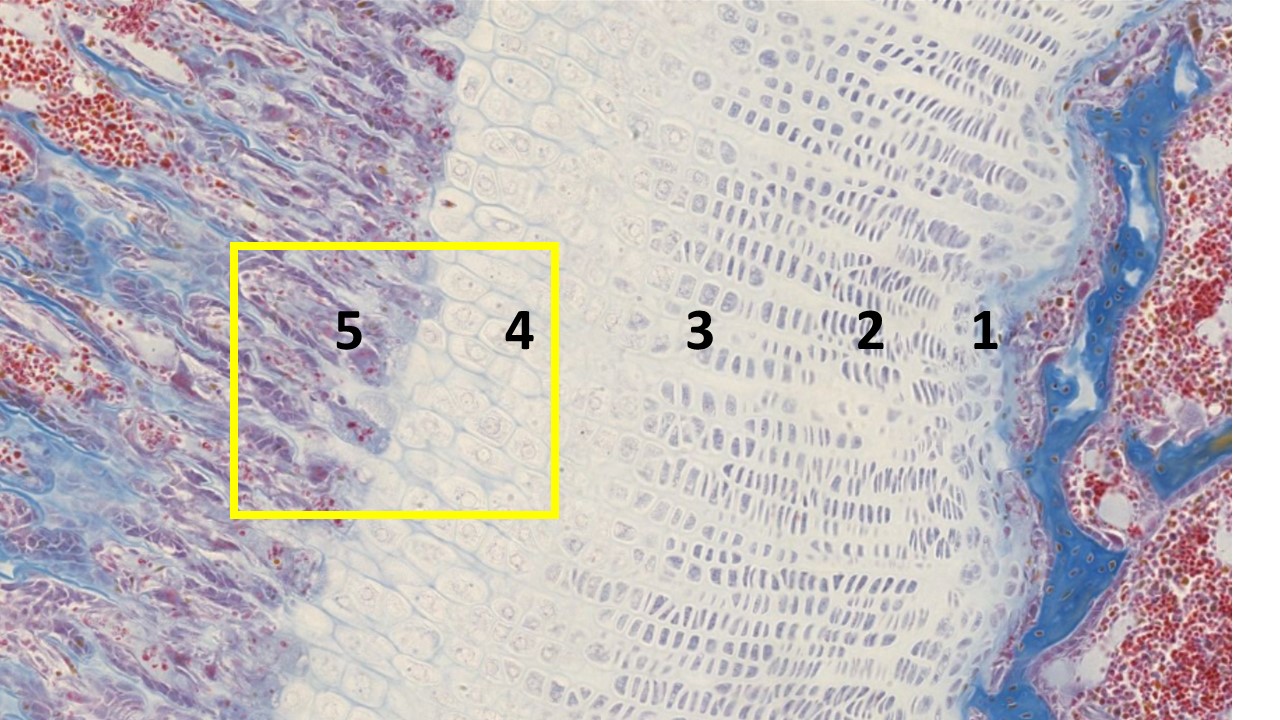
The image above shows endochondral bone formation. This region of the epiphyseal plate reflects the mechanism whereby the cartilaginous model of a long bone is converted to bone matrix and provides a mechanism for longitudinal bone growth. For orientation, the bits of blue material to the right represents bone at the base of the epiphysis. A band of cartilage consisting of anatomically and functionally distinct growth zones forms the epiphyseal plate.
- Zone 1 (resting zone) is considered to contain resting chondrocytes (they are not proliferating and have a random orientation within the cartilage matrix). This is typical hyaline cartilage.
- Zone 2 (proliferation zone) consists of chondrocytes undergo proliferation, indicated by the in stacks of chondrocytes being formed (isogenous groups). Because they are proliferating so rapidly in columns and secreting more collagen and proteoglycans, the cartilage (epiphyseal) plate lengthens toward the epiphyses.
- Zone 3 (hypertrophic zone) consists of large, swollen chondrocytes that are terminally differentiated. These chondrocytes compress the matrix into aligned spicules that are within a newly vascularized environment ready for calcification.
- Zone 4 (zone of calcified cartilage) marks the release of matrix vesicles and the protein osteocalcin from dying chondrocytes. Together, these structures begin the calcification of the organic matrix by aiding the formation of hydroxyapatite crystals.
- Zone 5 (zone of ossification) is when the first bone tissue appears. Osteoprogenitor cells invade via the capillary network of the epiphyseal plate and take up residence in the lacunae left behind from the dead chondrocytes and differentiate into osteoblasts. The osteoblasts line up along the spicules of calcified cartilage matrix and secrete the osteoid that will, in turn, become mineralized to form woven/primary bone. Later, the woven bone will be remodeled by the coordinated activities of osteoclasts and osteoblasts to form lamellar bone. With normal bone homeostasis, bone resorption occurs at a pace that bone formation can keep up to replace any bone that was digested away.
Optional activity:You can practice looking at endochondral ossification using a virtual microscope with guiding text in the right panel at this link.
Yellow Box From Micrograph Above: Higher Magnification of Epiphyseal Plate:
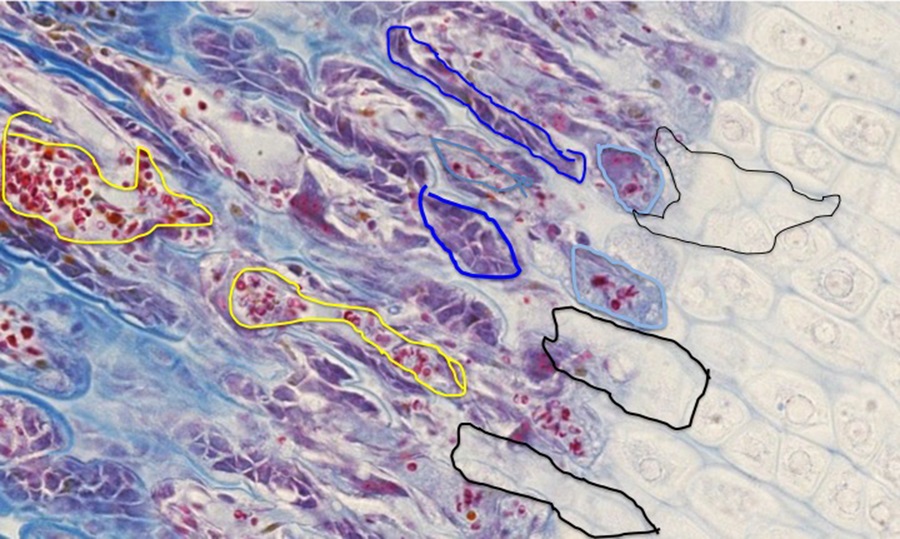
During the course of cartilage matrix mineralization, the chondrocytes die, leaving stacks of empty lacunae (areas outlined in black on image above). These spaces are invaded by small blood vessels (outlined in yellow) that bring with them the cellular elements of bone formation and remodeling-osteoprogenitor cells that differentiate into osteoblasts (outlined in dark blue) come in with the connective tissue associated with the blood vessels, and osteoclasts (light blue circles) arise from the differentiation of blood-borne monocytes.
If we shift our focus on this slide to the developing synovial joint that will allow for a range of motion between the femur (F), tibia (T), and patella (P; see lower magnification of the same micrograph below), there are several features of note:
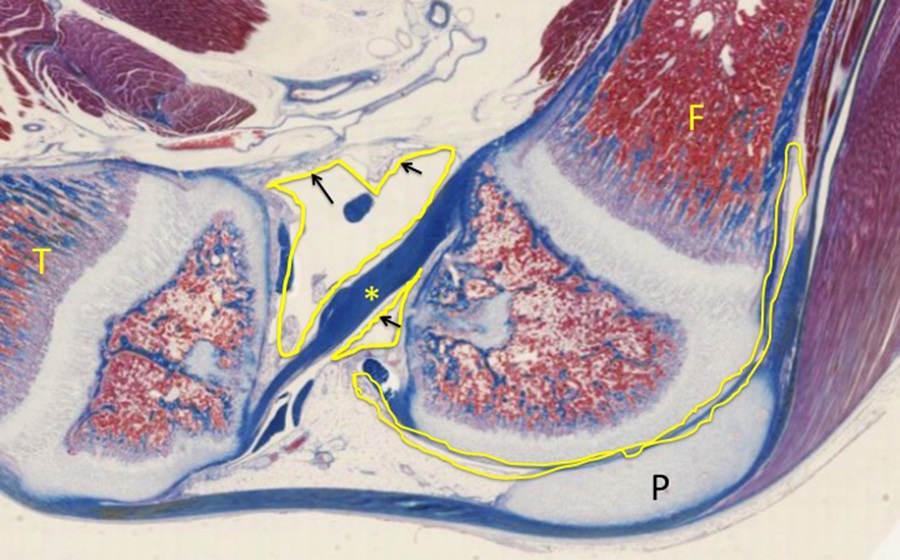
At areas of movement (articulation), hyaline cartilage will remain (articular cartilage). Review that in contrast to hyaline cartilage elsewhere in the body, articular cartilage does NOT have a covering of perichondrium. The joint space is sealed off by a collar of membrane (synovial membrane in yellow) that is reinforced during development by dense regular connective tissue, which contributes to the joint capsule. The cells of the synovium (black arrows) produce a viscous, synovial fluid that lubricates the joint. Asterisk indicates the anterior cruciate ligament of the knee joint.
INTRAMEMBRANOUS BONE FORMATION
Slide 5. Skull (Trichrome)

The formation and growth of flat bones, such as the skull bone displayed above, does not utilize a cartilaginous model. Instead, connective tissue cells become committed to an osteogenic lineage and begin to deposit bone matrix directly without the involvement of a cartilagenous model (area spanned by the thick black line above).
Higher Magnification of the Osteogenic Connective Tissue Area:
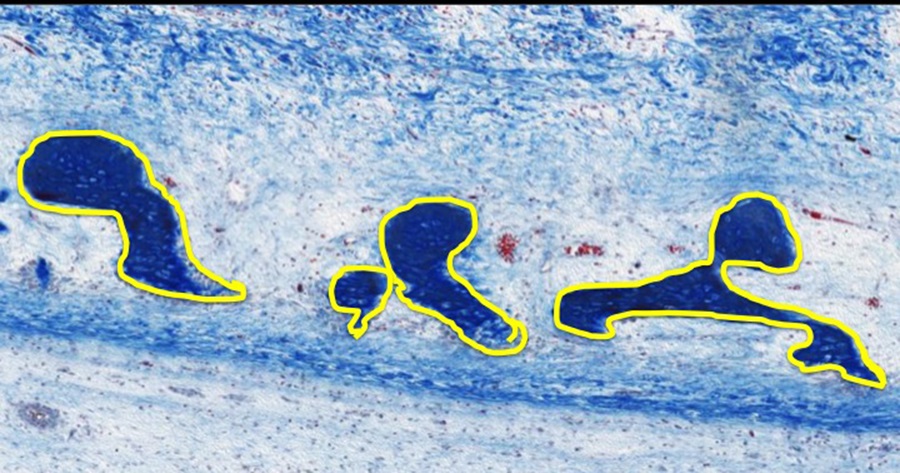
Osteogenic cells line the surfaces of the spicules of bone (outlined in yellow on image above) that are comprised of osteocytes and bone matrix.
Slide 6. Another example of intramembranous bone formation(Modified H&E)
The developing bone (see micrograph below) is highlighted with the yellow bracket, connective tissue highlighted with blue bracket, and epithelium (with hair shafts) highlighted in black bracket.

This sample is more developed than the one above in that the spicules of bone are more pronounced and interconnected, although sheets of dense bone have not yet formed on the surfaces.
Higher Magnifcation of Yellow Bracket Area From Micrograph Above:
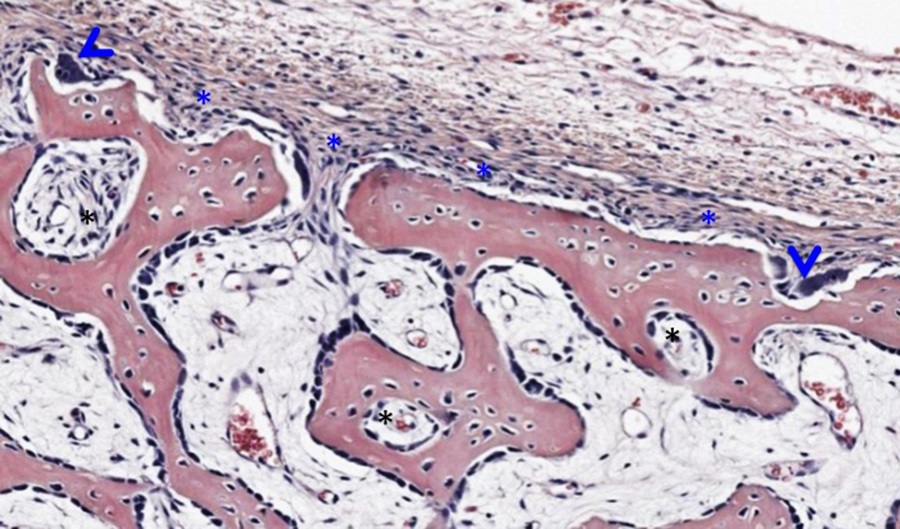
Examining the portion of bone indicated within the yellow bracket reveals a layer of periosteum (blue asterisks along the surface of the eosinophilic bone, image above). Two osteoclasts nestled in Howship’s lacunae are indicated by blue arrowheads. Black asterisks indicate developing haversian systems cut in cross section. Osteocytes are enclosed within lacunae and endosteum containing osteoblasts and osteoprogenitor cells cover the spicules of bone and line the immature osteons.
Optional activity: You can practice looking at intramembranous ossification using a virtual microscope with guiding text in the right panel at this link.
This Chapter’s PDF
- Note: The interactive features of this chapter are not reproducible in this PDF format.
ARCHIVAL PRE-CLASS MATERIALS FOR THIS SESSION:
- Pre-recorded videos: Bone

Feedback/Errata