1 Anatomy of the Basal Ganglia
Learning Objectives:
-
Describe the locations of the five nuclei that make up the basal ganglia, and describe their connections via the thalamus with cerebral cortex
-
Describe the direct and indirect pathways through the basal ganglia, noting the major transmitters used by each structure and the overall effect of activity in each pathway on thalamo-cortical activation
-
Compare the effects of damage to the basal ganglia, cerebellum, motor neurons and corticospinal tract on voluntary movement
-
Explain how input from the substantia nigra influences the direct and indirect pathways, and the role of dopamine in the pathways
Table of Contents:
Introduction:
Movement disorders, especially Parkinson disease, are a major source of disability in the aging population and are typically caused by dysfunction in the circuitry of the basal ganglia. Buried deep within the hemispheres of the brain, these nuclei are largely responsible for the initiation and regulation of movement. As discussed previously, control of muscle activity is directly exerted by descending motor pathways, consisting of upper motor neurons with cell bodies located in the motorcortex or brainstem, and lower motor neurons with cell bodies located in the anterior horns of the spinal cord that synapse directly on muscle. The basal ganglia modulate activity in the motor cortex indirectly via a thalamic relay. Because the basal ganglia do not communicate directly with motor neurons or receive feedback from the periphery, they are not involved with the control of ongoing movement. These functions depend on feedback from the periphery, and thus are served by the cerebellum.
General Anatomy:
The basal ganglia are a collection of nuclei situated deep in the cerebral hemispheres (just lateral to the thalamus) and in the rostral midbrain (Fig. 1). Overall, the basal ganglia consist of five paired nuclei:
1. Caudate: (“tail”)
2. Putamen: (“shell”)
3. Globus pallidus: (“pale ball”)
4. Substantia nigra: (“dark substance”)
5. Subthalamic nucleus: (“beneath the thalamus”)
Striatum = Caudate and Putamen
Lenticular Formation = Putamen and Globus Pallidus
Figure 1. Movie going through the brain to the nuclei of the basal ganglia
The putamen and the caudate nucleus, consisting of “head,” “body” and “tail,” are the primary input nuclei of the basal ganglia. Although functionally similar, these two structures are separated by the anterior limb of the internal capsule, with the caudate nucleus being medial to the internal capsule and forming part of the border of the lateral ventricle. Bridges of gray matter connect the two nuclei. These “striations”, or stripes, give rise to the name of corpus striatum (“body of lines”), which refers to both structures. The nucleus accumbens is the main area of contact between the two nuclei in the ventral forebrain and is involved with the limbic system and motivated behavior. The globus pallidus and the substantia nigra are the primary output nuclei of the basal ganglia. Notice in Fig. 2 that the putamen and globus pallidus together form what appears to be a “lens-like” structure, and thus are often referred to as the “lenticular nucleus.” Finally, the subthalamic nucleus is involved in internal circuitry of the basal ganglia.
Figure 2. Image sliders showing gross and MRI images in A) Coronal and B) Axial planes with showing nuclei of the basal ganglia.
Major pathways:
Overall the basal ganglia are involved in neural circuitry loops which originate in the cortex and return to the cortex via a thalamic relay. These circuits can be subdivided into four pathways: motor, oculomotor (Fig. 3), prefrontal (executive function and decision making), and limbic (motivation and emotional processing) (Fig. 4). The motor and oculomotor pathways are by far the best understood and will be the primary focus of this chapter.
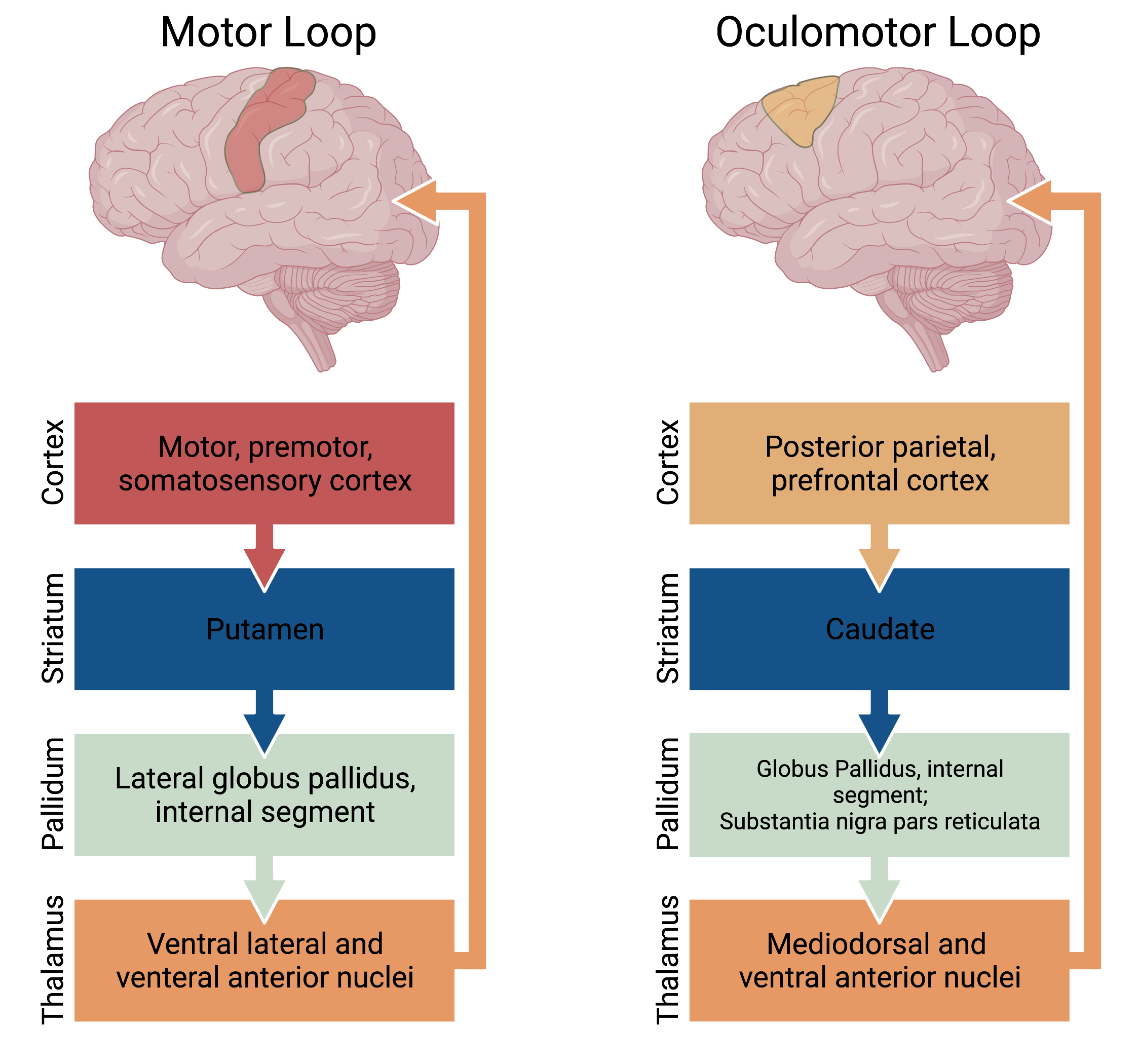 Figure 3. The “motor” systems of the basal ganglia consisting of the motor and oculomotor loops.
Figure 3. The “motor” systems of the basal ganglia consisting of the motor and oculomotor loops.
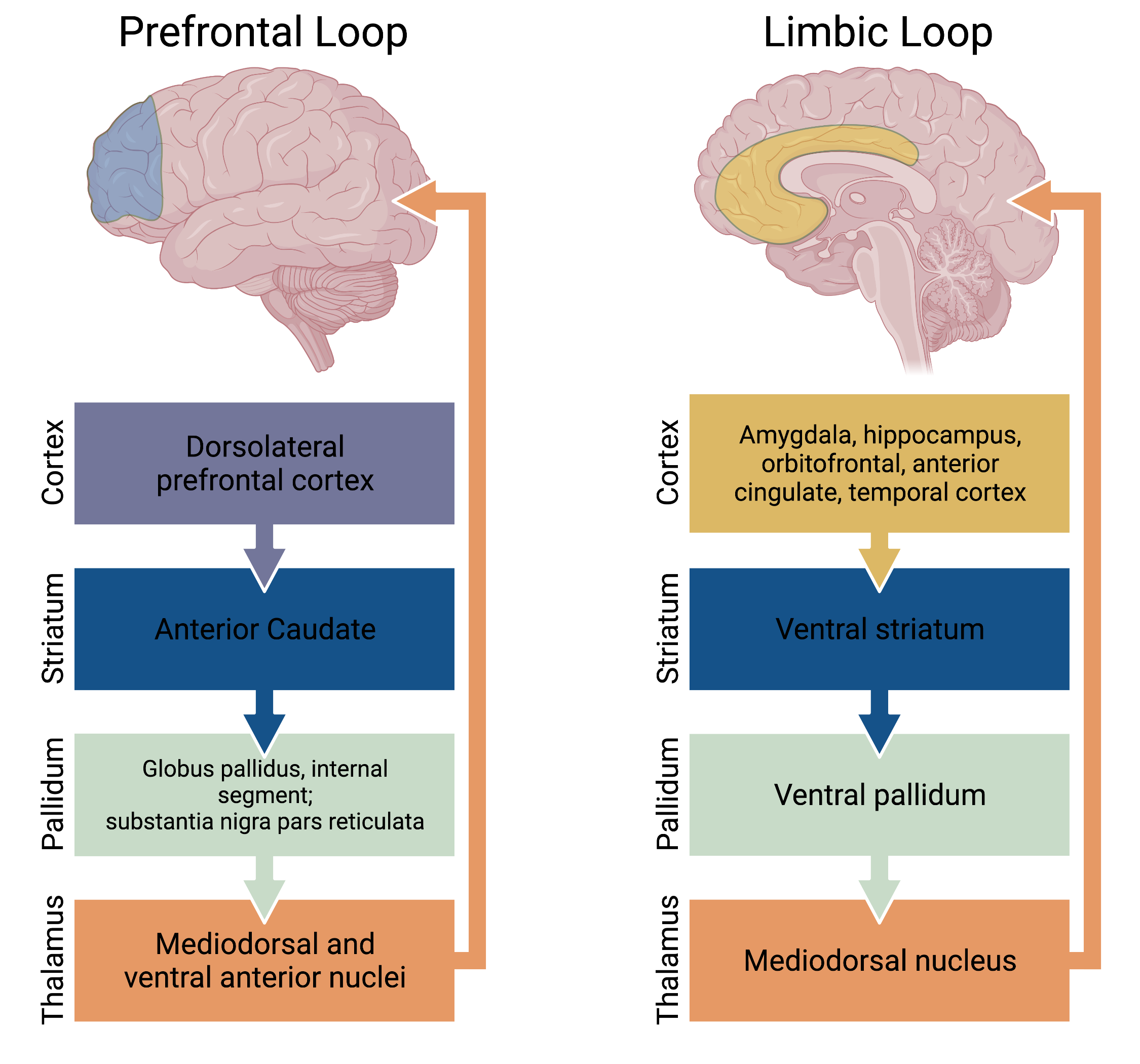
Figure 4. The “non-motor” systems of the basal ganglia consisting of the prefrontal and limbic loops.
Inputs:
The caudate and putamen receive excitatory glutamatergic input from widespread cortical and subcortical structures, except the primary visual and auditory cortices. The putamen is primarily involved in motor pathways, and receives input from the premotor and motor cortices. The caudate nucleus is primarily involved in oculomotor and prefrontal pathways, and receives input from the frontal eye fields, frontal, parietal, and motor cortices. The nucleus accumbens plays a role in the limbic pathway, and receives the majority of its input from the temporal cortex, the amygdala, and the hippocampus.
The majority of caudate and putamen neurons send GABAergic projections to different regions of the globus pallidus and substantia nigra pars reticulata (SNr).
Another important input to the basal ganglia arrives via the nigrostriatal pathway. This projection originates in the dopaminergic cells of the substantia nigra pars compacta (SNc) in the midbrain. A parallel dopaminergic projection to the nucleus accumbens originates in the ventral tegmental area (VTA), which is medial to the substantia nigra in the midbrain. As discussed below, dopamine can either have an excitatory or inhibitory effect depending on the specific receptor on the post-synaptic cell.
Outputs:
All outputs from the basal ganglia are inhibitory and most originate in GABAergic neurons located in either the internal segment of the globus pallidus (GPi) or the substantia nigra pars reticulata (SNr). In general, outputs related to motor control of the head and neck originate in the SNr, and outputs related to motor control for the rest of the body originate in the GPi. The majority of the outputs from the basal ganglia reach the ventrolateral (VL) and ventroanterior (VA) thalamic nuclei. From here, the outputs project to nearly the entire frontal lobe. Both the GPi and SNr send projections to the pontomedullary reticular formation of the midbrain.
The Motor Pathways of the Basal Ganglia:
Control of movement by the basal ganglia is exerted via modulation of excitatory drive from the thalamus to the motor cortex. Additional outputs target locomotion centers in the midbrain, which drive gait via the reticulospinal tract. In the classical model, activation of the “direct” pathway facilitates intended movements, while activation of the “indirect” pathway acts in a complementary manner to refine movement by inhibiting similar, but unintended, movements.
Output neurons of the basal ganglia are tonically active, and thus provide a constant inhibitory tone on their targets – excitatory neurons of the thalamus. These excitatory neurons, in turn, project to upper motor neurons in the motor cortex. Thus, the inhibitory output of the basal ganglia holds voluntary movements of the limbs in check. As described below, activation of the direct pathway interrupts this tonic inhibitory output, a process known as disinhibition, thereby releasing excitatory output of the thalamus to facilitate movement. Activation of the indirect pathway also uses the process of disinhibition to refine movement by inhibiting undesired movements.
The primary neuron of the striatum is the medium spiny neuron (MSN). These inhibitory GABAergic neurons comprise almost 90% of the neurons in the striatum, and each MSN receives input from many cortical pyramidal cells and dopaminergic neurons from the SNc. The activity of striatal MSNs is also enhanced by excitatory input from local cholinergic interneurons.
The Direct Pathway:
The primary purpose of the direct pathway is to facilitate initiation of voluntary movements. This is accomplished by a process of disinhibition, which modulates the activity of the motor cortex by releasing excitatory drive from the VA/VL nuclei of the thalamus. To initiate a movement, the cerebral cortex excites the striatum. This excites the direct pathway, which has GABAergic projections (also express D1 dopamine receptors) to the globus pallidus internal (GPi) segment and inhibits its activity. The resulting inhibition produces a pause in the tonic inhibitory outputfrom the GPi neurons. The resulting disinhibition of their target neurons in the VA/VL nuclei of the thalamus increases excitatory drive onto upper motor neurons in the motor cortex via a glutamatergic project and helps initiate a desired movement.
Figure 5. The direct pathway image blender. Slide across the bottom to go through the pathway.
The Indirect Pathway:
The primary purpose of the indirect pathways to refine voluntary movement and to prevent unwanted movement. Thus, the indirect pathway is activated concurrently with the direct pathway and acts in a complementary fashion. The indirect pathway also uses the process of disinhibition to accomplish this goal, but the targets of disinhibition are the excitatory neurons of the subthalamic nucleus, which project to the GPi. Thus, activation of the indirect pathway enhances the inhibitory output of the GPi. Similar to the direct pathway, to initiate a movement, the cerebral cortex excites the striatum. This also excites striatal neurons in the indirect pathway. These GABAergic neurons (also express D2 dopamine receptors) inhibit the globus pallidus external (GPe) segment. Neurons of the GPe send tonically active GABAergic inhibitory projections that target excitatory neurons in the subthalamic nucleus (STN). These, in turn, send a glutamatergic excitatory projection to the GPi. The overall effect of the indirect pathway is to enhance inhibitory output of the GPi and decrease the excitatory output of the VA/VL nucleus of the thalamus, thereby blocking unwanted movements.
Figure 6. The indirect pathway image blender. Slide across the bottom to go through the pathway.
Figure 7. The direct and indirect pathway image slider.
Movement is controlled by synergistic activity in both pathways:
The two pathways work together by helping initiate the desired movement (direct pathway) while inhibiting associated undesired movement (indirect pathway). The dopaminergic nigrostriatal pathway: As mentioned above, striatal neurons receive dopaminergic inputs from neurons in the SNc via the nigrostriatal tract. The substantia nigra (“black substance”) receives its name from the dark pigmentation due to the presence of neuromelanin, which is formed from the dopamine precursor, L-DOPA. Depending on the type of post-synaptic receptor, dopamine release can elicit either an excitatory or inhibitory response in the post-synaptic cell. Striatal neurons of the direct pathway express D1 dopamine receptors, which when stimulated increase neuronal excitability; in contrast, striatal neurons of the indirect pathway express D2 dopamine receptors, which decrease neuronal excitability. The release of dopamine by the nigrostriatal tract simultaneously enhances activity in the direct pathway and reduces activity in the indirect pathway. Thus, overall, dopamine facilitates the initiation of voluntary movement.
TL/DR:
Nuclei of the basal ganglia:
1. Caudate 2. Putamen 3. Globus pallidus 4. Substantia nigra 5. Subthalamic nucleus
Striatum = Caudate and Putamen
Lenticular Formation = Putamen and Globus Pallidus
Direct and indirect pathways:
Direct – facilitates movement through disinhibition of the thalamus ( + – – +) = movement
Indirect – prevents unwanted movement by inhibiting the thalamus ( + – – + – -) = no movement
Substantia Nigra Dopamine:
Direct Pathway = D1 receptor = excitatory
Indirect Pathway = D2 receptor = inhibitory
