1 The TCA cycle
The TCA Cycle
(rev. 10/28/2022)
This session continues our study of metabolism. After the initial catabolism of glucose by glycolysis, what further processing happens? When oxygen is present, how can catabolism capture more energy in the form of ATP? How does metabolism produce more metabolic intermediates essential for synthesizing other molecules (anabolism)?
Session Learning Objectives:
4. Explain how the TCA cycle is regulated, including which critical steps are regulated.
Goals:
To provide an understanding of the catabolism of glucose under aerobic conditions. We will discuss the importance of the pyruvate dehydrogenase reaction as a first step for acetyl-CoA entry into the TCA cycle. We will describe the cycle itself, its regulation, and the shuttling of electrons through the electron transport chain (ETC) to generate ATP.
SLO1: 1. Explain the significance of the TCA cycle and its biological roles, including its role in catabolic and anabolic pathways.
Definitions:
TCA Cycle: tricarboxylic acid cycle. It is also called interchangeably the “citrate cycle” or the “Krebs cycle.” It gets these various names because the pathway is cyclic, was described by Sir Hans Krebs, and a key intermediate compound is citric acid, which is a 6-carbon tricarboxylic acid.
Oxidation and reduction. Oxidation is the abstraction of electrons from a molecule, which we refer as the electron donor molecule; and reduction is the acceptance of electrons by a molecule previously in a more oxidized state, which we refer to as the electron acceptor molecule. Oxidation-reduction reactions are always coupled. An oxidizing agent abstracts electrons from an electron-rich reducing agent. Common examples: Oxygen oxidizes elemental iron Fe° by removing electrons to yield Fe2+ (rust) and it oxidizes glucose to CO2 and H2O. Dehydrogenase enzymes catalyze oxidations by removing electrons from their substrates often transferring them to NAD+ or NADP+ forming NADH or NADPH. NADPH is a reducing agent. We say that NADPH has reducing power since it can transfer electrons to a molecule that is oxidized and can accept electrons; in the process NADPH becomes oxidized back to NADP+. A similar situation arises for NAD+ forming the reducing agent NADH. However, remember that NAD+/NADH and NADP+/NADPH are used for different purposes in metabolic pathways.
What does it do?
The TCA cycle intersects with the electron transport chain (ETC) to make many molecules of ATP from the oxidation of carbon substrates derived from glucose or fat. These processes are aerobic meaning that oxygen is consumed. After glycolysis has generated pyruvate (3-carbons), pyruvate is oxidatively decarboxylated by Pyruvate Dehydrogenase (PDH) to form acetyl-coA which then enters the TCA cycle. Through a sequence of steps, The TCA cycle fully oxidizes acetyl-CoA to CO2 while generating energy in the form of reduced NADH and electrons. The electrons of NADH are then transferred to the ETC through Complex I to generate ATP. In parallel, the electrons of cytosolic NADH are passed on via a series of steps involving the malate-aspartate shuttle to the mitochondrial matrix, generating NADH in the matrix, which is used for ATP production via the ETC.
Several of the molecules generated by the TCA cycle also can be tapped as building blocks for synthesis of other key metabolic intermediates including amino acids. The core enzymes of both the TCA cycle and of the ETC are located inside of mitochondria. Aerobic glucose catabolism is particularly important for Type I skeletal fibers, the heart, and brain function, tissues that consume a lot of O2. Overall, ~ 36 ATPs are produced, and 2 ATPs are consumed per molecule of glucose catabolized under aerobic conditions–including those few from glycolysis. This process involves communication between glycolysis in the cytosol and the TCA cycle, electron transport, and oxidative phosphorylation inside the mitochondrial matrix.
SLO 2. Explain how the irreversible reaction catalyzed by the pyruvate dehydrogenase complex leads to the entry of acetyl-CoA into the TCA cycle and why acetyl-CoA cannot be used as a substrate for gluconeogenesis.
Pyruvate entry into the TCA cycle:
The pyruvate produced through glycolysis is transported into the mitochondria, where it is oxidized to acetyl CoA, by action of pyruvate dehydrogenase, and then enters the TCA cycle. The PDH reaction is critical, as it is irreversible and ensures that acetyl-coA cannot be used in the reverse reaction for gluconeogenesis. While acetyl-CoA is not a substrate for gluconeogenesis, its oxidation through the TCA cycle and electron flow through the ETC produces the energy (ATP) necessary for gluconeogenesis.
Note that PDH is an enzyme complex, is comprised of three enzyme that catalyze different steps of the reaction. The PDH complex requires 5 cofactors for proper function which are: Thiamine pyrophosphate (TPP); Lipoic Acid; Coenzyme-A; NAD+/NADH; and FAD/FADH2.
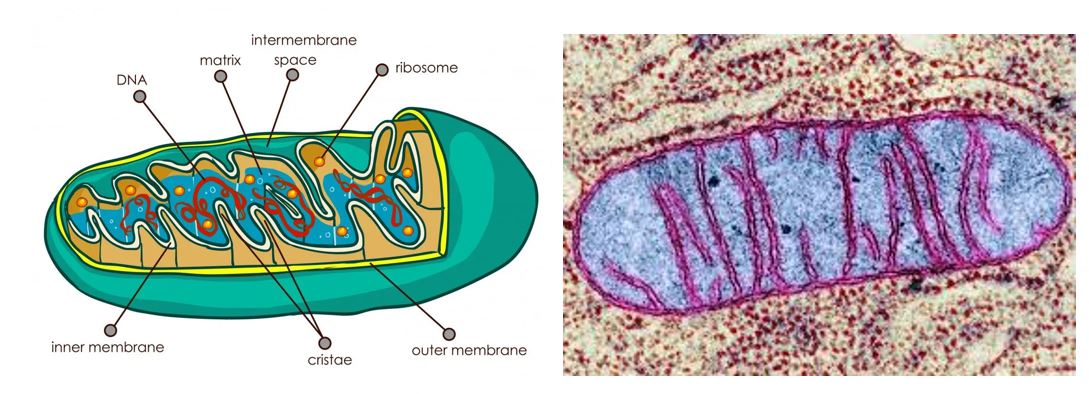
Figure 1. The Mitochondrium. Left, Cartoon of mitochondria with key features and histology. Mitochondria are spatially divided into an intermembrane space and inner matrix by the inner membrane. The matrix contains mitochondrial genomes and ribosomes. Right, pseudocolored electron micrograph of a mitochondrium.
Mitochondria: Mitochondria are cytoplasmic organelles richly endowed with many biochemical pathways. Mitochondria is a plural word. The singular is mitochondrion. The mitochondrial matrix contains the enzymes of the TCA cycle, fatty acid oxidation and the urea cycle, as well as the machinery for replicating and expressing mitochondrial DNA. The outer membrane contains pores which allow free diffusion of small molecules. In contrast, the inner membrane forms a tight barrier and contains specific transporters for small molecules such as pyruvate and malate. The inner mitochondrial membrane contains the electron transport system as well as the proteins involved in oxidative phosphorylation. In sum, mitochondria are discrete structures dedicated to energy production (ATP), but they are also the sites of other metabolic processes including beta-oxidation of fatty acids, initiation of the urea cycle (part of nitrogen metabolism), and part of the heme biosynthetic pathways.
Figure 2. The steps of the TCA cycle:
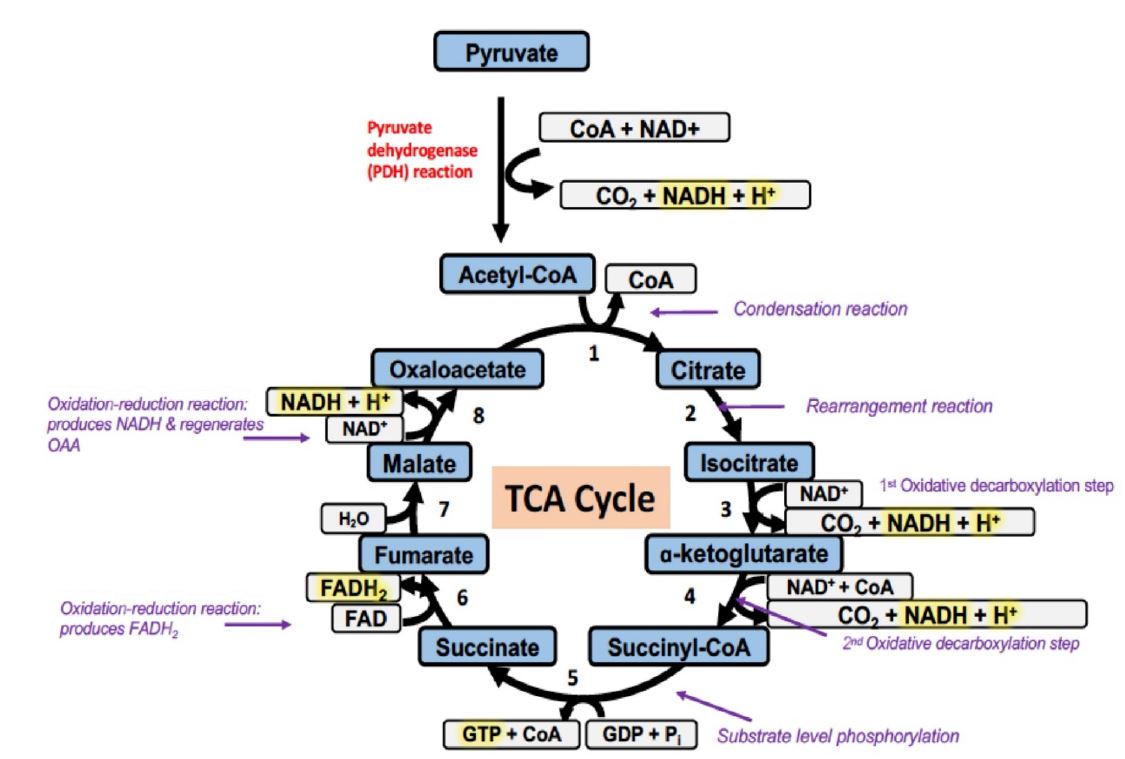
The key steps of the TCA cycle are catalyzed by the following enzymes:
Reaction 1: citrate synthase (regulated step)
Reaction 2: aconitase
Reaction 3: isocitrate dehydrogenase (regulated step & generate energy in the form of NADH)
Reaction4: α-ketoglutarate dehydrogenase (regulated step & generate energy in the form of NADH)
Reaction 5: succinyl-CoA synthase
Reaction 6: succinate dehydrogenase (generates energy in the form of FADH2)
Reaction 7: fumarase
Reaction 8: malate dehydrogenase (generates NADH when malate is oxidized to oxaloacetate)
SLO3. Outline the co-factor requirements for the pyruvate dehydrogenase and alpha ketoglutarate dehydrogenase enzymes and the biological consequences of thiamine deficiency.
Definitions:
Oxidative decarboxylation: A complex reaction that cleaves carboxylic acid off a carbon substrate, releasing CO2, and deriving much energy in the form of reduced cofactors. The carbon substrate loses one carbon and is oxidized while the cofactor is reduced.
Pyruvate Dehydrogenase Complex (PDH): A key step prior to entry of acetyl-CoA into the TCA is the oxidative decarboxylation of pyruvate to acetyl-CoA, resulting in the formation of NADH, acetyl-CoA and the loss of one CO2 molecule. Thus the 3-carbon pyruvate molecule is split in a 2-carbon (acetyl) and 1-carbon (CO2) fragment. PDH is a multi-enzyme complex that requires important cofactors for its function. These are thiamine pyrophosphate (derived from thiamine/vitamin B1), lipoic acid, coenzyme-A, FAD/FADH2, and NAD+/NADH. Like PDH, α-ketoglutarate dehydrogenase in the TCA cycle is a similar multi-enzyme complex requiring the same 5 cofactors for proper function.
Thiamine Deficiency: Thiamine deficiency causes beriberi, which most dramatically affects the nervous system and the heart. This is because these two tissues are highly dependent on oxidative metabolism for energy production. Beriberi is a problem in those parts of the world where rice is a major food, since rice has a particularly low thiamine content. In the USA, beriberi is most common in individuals with poor nutrition, high ethanol intake (which leads to malabsorption of thiamine),or severe kidney disease. It is characterized by abnormalities in the peripheral nervous system, reflected in abnormal sensation and muscular weakness, and an enlarged heart.
Regulation of PDH. Control of PDH activity is important, because the reaction is irreversible and commits pyruvate either to complete oxidation through the TCA cycle or to the products derived from acetyl-CoA(for example fatty acids, steroids or ketone bodies; discussed later in the course). PDH is regulated by phosphorylation and de-phosphorylation catalyzed by kinase and phosphatase enzymes, respectively. Formation of the inactive (phosphorylated) state is stimulated by high ATP levels and also by the products of the PDH reaction, acetyl-CoA and NADH. Hence, when energy charge is high and/or the TCA cycle is saturated with acetyl-CoA, the key reaction catalyzed by PDH is turned off.
SLO4. Explain how the TCA cycle is regulated, including which critical steps are regulated.
Figure 3. Molecules that regulate the TCA cycle (ADP, Ca2+, acetyl CoA, pyruvate, NAD+, CoA, NADH, citrate).
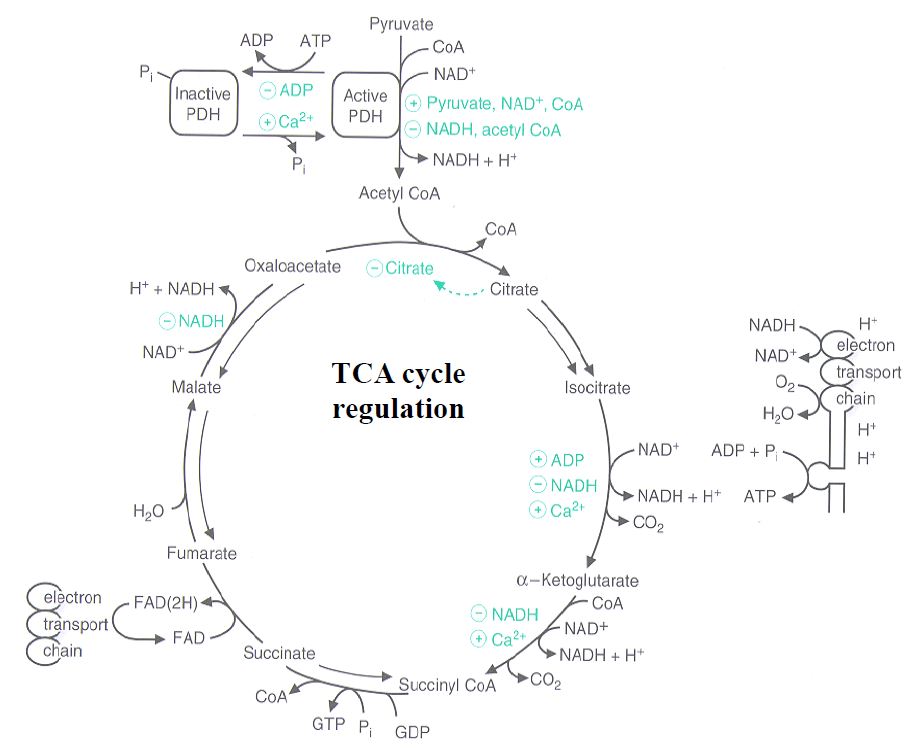
Regulation of the TCA cycle: The TCA cycle is regulated at two steps, catalyzed by isocitrate dehydrogenase (reaction 3) and α-ketoglutarate dehydrogenase (reaction 4). In general, these reactions are regulated by energy charge and by the ratio NAD+/NADH. Because of the tight regulation of PDH, the rate of the citrate synthase reaction can often be limited by the availability of acetyl-CoA. In addition to regulation by the NAD+/NADH ratio, α-ketoglutarate dehydrogenase is also activated by Ca2+. Calcium activation of this enzyme (together with activation of the PDH reaction) helps drive ATP production at times of increased Ca2+ flux inside the cell, including muscle contraction and hormone secretion which are energetically expensive.
SLO5 Explain how the TCA cycle can be used for synthesis of intermediates involved in biosynthetic reactions, and how pyruvate carboxylase replenishes oxaloacetate for TCA cycle activity.
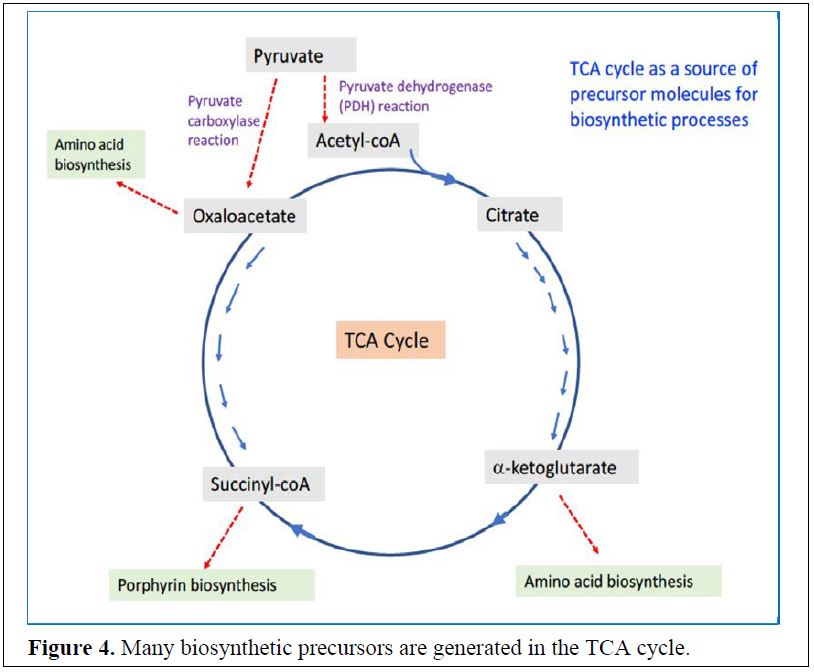
The TCA cycle also provides intermediates for biosynthetic processes: Besides being the major path-way for the oxidative generation of ATP, the TCA cycle also provides intermediates for biosynthetic reactions. For example, many amino acids can be derived from oxaloacetate and α-ketoglutarate if needed, and many of the carbon atoms of the porphyrin ring are derived from succinyl-CoA (Figure 3). The reverse is also true as these TCA cycle intermediates can be replenished by metabolism of amino acids.
Replenishing the TCA cycle of intermediates – The pyruvate carboxylase reaction replenishes the TCA cycle with OAA (Oxaloacetate)
The reaction catalyzed by pyruvate carboxylase. If intermediates are drained out of the TCA cycle for biosynthetic purposes, how is the oxaloacetate that is required to run the TCA cycle regenerated? This is accomplished by the enzyme pyruvate carboxylase that we will see is also key for gluconeogenesis.
Pyruvate + C02 + ATP -> Oxaloacetate + ADP +Pi
This enzyme requires biotin (vitamin B7), and the reaction is an energy dependent process needing ATP. The reaction, termed an anaplerotic (replenishing) reaction, is defined as forming an intermediate for a metabolic pathway. Here it replenishes oxaloacetate needed by the TCA cycle. When oxaloacetate is limiting for the first step of the TCA cycle, acetyl-CoA builds up. The elevated acetyl-CoA allosterically activates pyruvate carboxylase, which in turn provides the needed oxaloacetate for the TCA cycle.
Practice questions to better understand TCA cycle:
1.What is the functional role of the TCA cycle under catabolic (degradation) conditions? Answer 1.
2.Which reactions are the most important and need to be regulated? Answer 2.
3.What would you expect in a patient who is thiamine (vitamin B1) deficient? (i.e., What symptoms would this patient have and what would happen biochemically?) Answer 3.
What would be the consequences of not being able to convert pyruvate to acetyl-CoA as a result of TCA cycle impairment?
4. How are intermediates of the TCA cycle replenished? Answer 4.
5. Why does the TCA cycle take so many steps just to decarboxylate acetate (starting with acetyl-CoA), as it would seem that converting the 2-carbon acetyl CoA into two 1-carbon CO2’s could be done with fewer reaction steps. Answer 5.
TCA Knowledge Quiz 1: Three quizzes in one: Names of key TCA molecules, products of the TCA cycle and carbon tracking. You do not need to fill every box to check your answers.
The TCA cycle produces reducing power (NADH, FADH2), GTP and metabolic intermediates.
Citrate synthase (promoted by acetyl CoA, inhibited by citrate)
Isocitrate dehydrogenase (NADH production; promoted by ADP, Ca2+; inhibited by NADH)
α-ketoglutarate dehydrogenase (promoted by Ca2+; inhibited by NADH). Requires B1, B2, B3, B5 and lipoic acid/thiamine, FADH2, NADH, Coenzyme A and lipoic acid).
Succinate dehydrogenase (produces FADH2)
Malate dehydrogenase (produces and inhibited by NADH).
Thiamine is essential for the function of pyruvate dehydrogenase complex (PHD) and alpha-ketoglutarate complex. This will result in an increase in pyruvate, which can be converted to lactic acid. This can result in metabolic lactic acidosis and lack of energy from metabolism of glucose.
Metabolism of some amino acids and fatty acids can replenish components of the TCA cycle. For example alpha-ketoglutarate is the keto form of the amino acid glutamate. Odd chain fatty acids are converted into succinyl CoA in a process that requires B12 in a process we will cover more in the single carbon metabolism session.
The steps of the TCA cycle oxidizes the carbons added to oxaloacetate to generate energy, produce and utilize metabolic intermediates and reducing power that will be harvested in the electron transport chain.

