1 Nociceptors
Learning Objective 1: To know how nociceptors are activated and sensitized.
Nociceptors are classified by the type of physical stimuli (i.e., modality) that they respond to. Some nociceptors respond to more than one modality and are referred to as polymodal nociceptors. Others, called sleeping or silent nociceptors, normally do not respond to any type of stimulus, but become activated under conditions of inflammation or tissue damage.
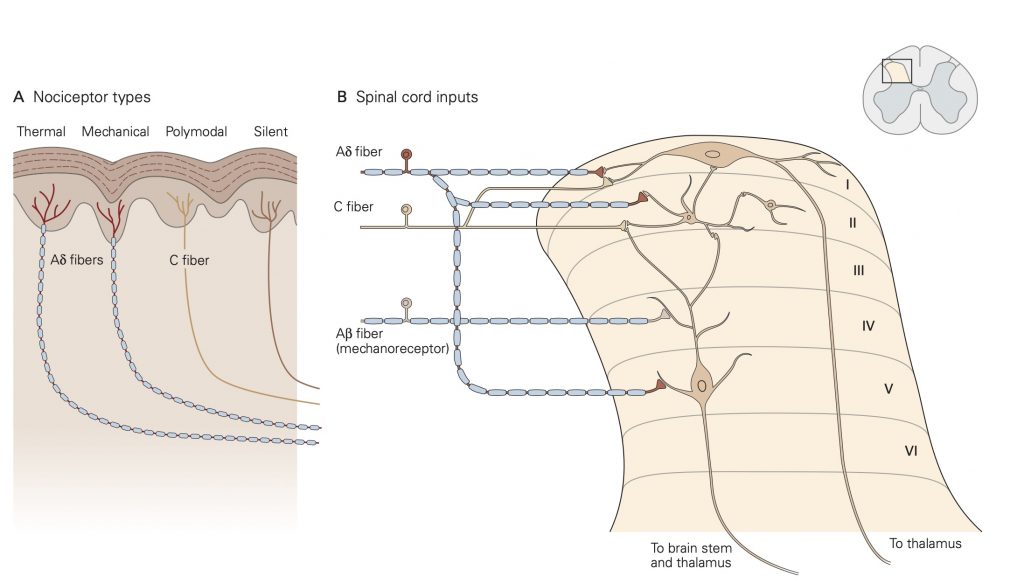
Thermal nociceptors are activated by noxious (i.e., tissue damaging) hot or cold temperatures within the receptive field acting on TRP channels. TRPV1 channels are expressed on thermal nociceptors that respond to heat/pain temperatures above 42 °C. Other temperatures in the warm–hot range are transduced by different thermal nociceptors that express a different TRP channel. Cold stimuli are sensed by thermal nociceptors that express TRPM8 channels. An interesting finding related to cold stimuli is that tactile sensibility and motor function deteriorate while pain perception persists.
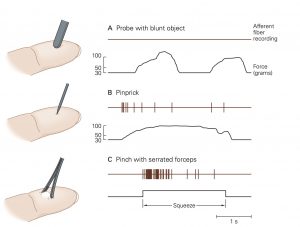
Mechanical nociceptors respond to excessive mechanical deformation of the tissue they innervate. They also respond to wounds that break the epidermal surface. These mechanical nociceptors that also express TRP channels frequently have polymodal characteristics, responding to thermal stimuli and chemical stimuli.
Chemical nociceptors express TRP channels that respond to a wide variety of molecules including spices like capsaicin (8-methyl-N-vanillyl-6-nonenamide), the active, hot sensation producing substance in chili peppers and environmental irritants like acrolein, a component of cigarette smoke. Chemical nociceptors also respond to endogenous ligands, and certain fatty acid amines that arise from damage to internal tissues.
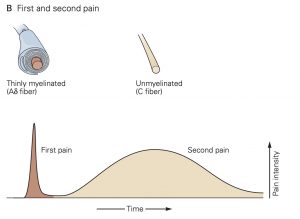
Individual nociceptors are grouped into two different classes, which have distinct types of axons: A-∂ fibers are myelinated and conduct action potentials quickly, within 10-30 m/s, while unmyelinated C-fibers conduct action potentials more slowly, from 0.5 – 2 m/s. Due to these differences in axonal conduction speed, nociception and the affective perception of pain often come in two phases: The first phase, initial sharp and localized pain, is mediated by the fast-conducting Aδ fibers and the second phase, continuous, long-lasting and more diffuse pain, is generated by activation of the slower- conducting C-fibers.
Response of nociceptors to tissue damage
Exposure to noxious stimuli often results in tissue damage. When this occurs, the plasma membrane of individual cells is breached and proteases are released. Proteases cleave proteins and produce peptides, including one termed bradykinin, a very potent pain-inducing substance. Cleaved bradykinin acts by binding to its receptors: B2 and B1. B2 receptors are constitutively expressed and are important in mediating pain detection and vasodilation. B1 receptors are upregulated in damaged tissue and play an important role in chronic pain. Bradykinin receptors on nerve terminals, especially free nerve endings, will depolarize in response to bradykinin binding.
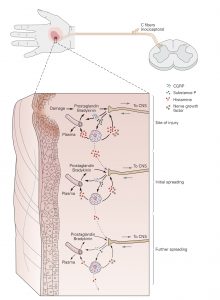
Sensitization of nociceptors
The responsiveness of nociceptors to stimulation can be modified, a process called sensitization, by a number of substances that interact with the receptive membrane. In the extreme, a nociceptor can be transformed from a noxious stimulus detector into one that responds to innocuous stimuli as well. When sensitized in this way, low intensity, innocuous stimuli associated with regular activity, can activate nociceptors and thus, generate a painful sensation. This is commonly known as hyperalgesia. Inflammation is one common cause of nociceptor sensitization. Normally hyperalgesia desists when the inflammation is resolved. Occasionally, however, repeated injury or chronic use of opioid analgesics can result in allodynia, a condition in which a completely non-noxious stimulus like light touch causes extreme pain. Allodynia can also be caused when nociceptor afferent fibers are damaged in the peripheral nerves.
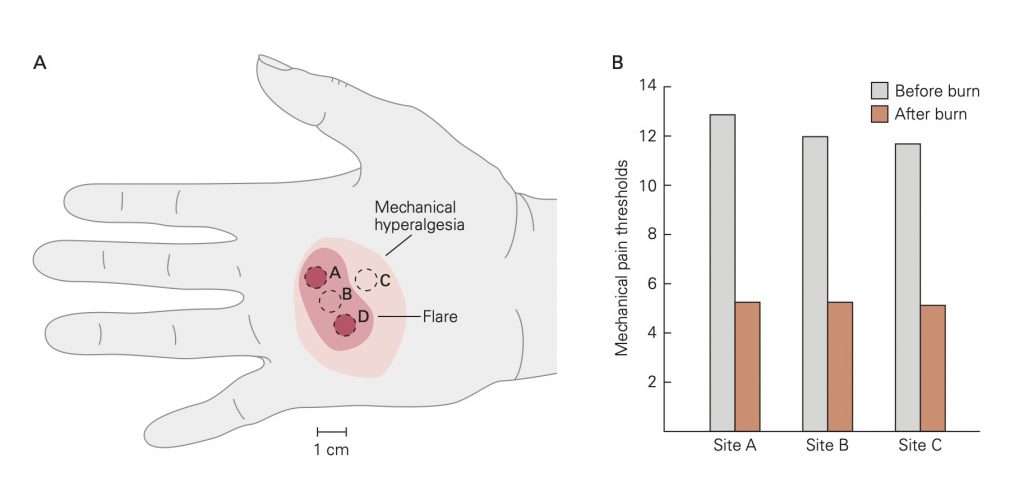
A. Mechanical thresholds for pain were recorded at sites A, B, and C before and after burns at sites A and D. The areas of reddening (flare) and mechanical hyperalgesia resulting from the burns are shown on the hand of one subject. In all subjects the area of mechanical hyperalgesia was larger than the area of flare. Mechanical hyperalgesia was present even after the flare disappeared.
B. Mean mechanical pain thresholds before and after burns. The mechanical threshold for pain is significantly decreased after the burn.
