Sensory Receptors
John Tuthill
Introduction
Sensory receptors are specialized, excitable cells that are designed to convert a physical stimulus into an electrical signal that can be transmitted to other nerve cells in the nervous system. The physical stimulus interacts with a specialized receptor protein embedded in the membrane of the sensory receptor leading to a change in membrane potential. Sensory receptors typically respond to only one type of stimulus and that specificity is conserved as the signal is propagated along neural pathways in the spinal cord and brain. The series of neurons that are connected by synapses from the sensory receptor to secondary sensory and third order sensory neurons in the nervous system is referred to as a ‘labeled line’. The labeled lines enable the brain to distinguish and process information about different types physical stimuli. In response to a constant stimulus, the response most sensory receptors declines over time, as process called adaptation. Some sensory receptors adapt slowly to a constant stimulus and others adapt more rapidly.
Learning Objectives
- To know the general properties of sensory receptors.
- To understand the labeled-line principle of signal detection.
- To compare the mechanisms of sensory transduction in different types of sensory receptors.
- To appreciate how the intensity and duration of a stimulus are reflected in the receptor potential and action potential discharge rate of a sensory neuron.
- To understand how sensory receptors adapt to a constant stimulus.
(Unless otherwise noted, all figures are from: Kandel ER, Schwartz JH, Jessell TM 2012, Siegelbaum SA, Hudspeth AJ. ‘Principles of Neural Science, 5th ed. McGraw-Hill, New York.)
Learning Objective #1: To know the general properties of sensory receptors.
Sensory receptors are specialized, excitable cells that are designed to convert a physical stimulus in the external world (e.g., light, sounds, odors, etc.) or one that occurs inside our bodies (e.g., blood pressure, muscle force, osmolality, etc.) into an electrical signal that can be transmitted to other nerve cells in the nervous system. In all cases, the physical stimulus interacts with a specialized receptor protein embedded in the membrane of the sensory receptor leading to a change in membrane potential. The receptor proteins in sensory receptors typically respond to only one type of stimulus that confers selectivity and specificity to the receptor. For example, the receptor protein in a cutaneous mechanoreceptor responds to the mechanical deformation of its membrane, whereas the receptor protein of a rod or cone photoreceptor in the retina responds to light energy. The form of energy that a receptor preferentially responds to is called the ‘adequate stimulus’.
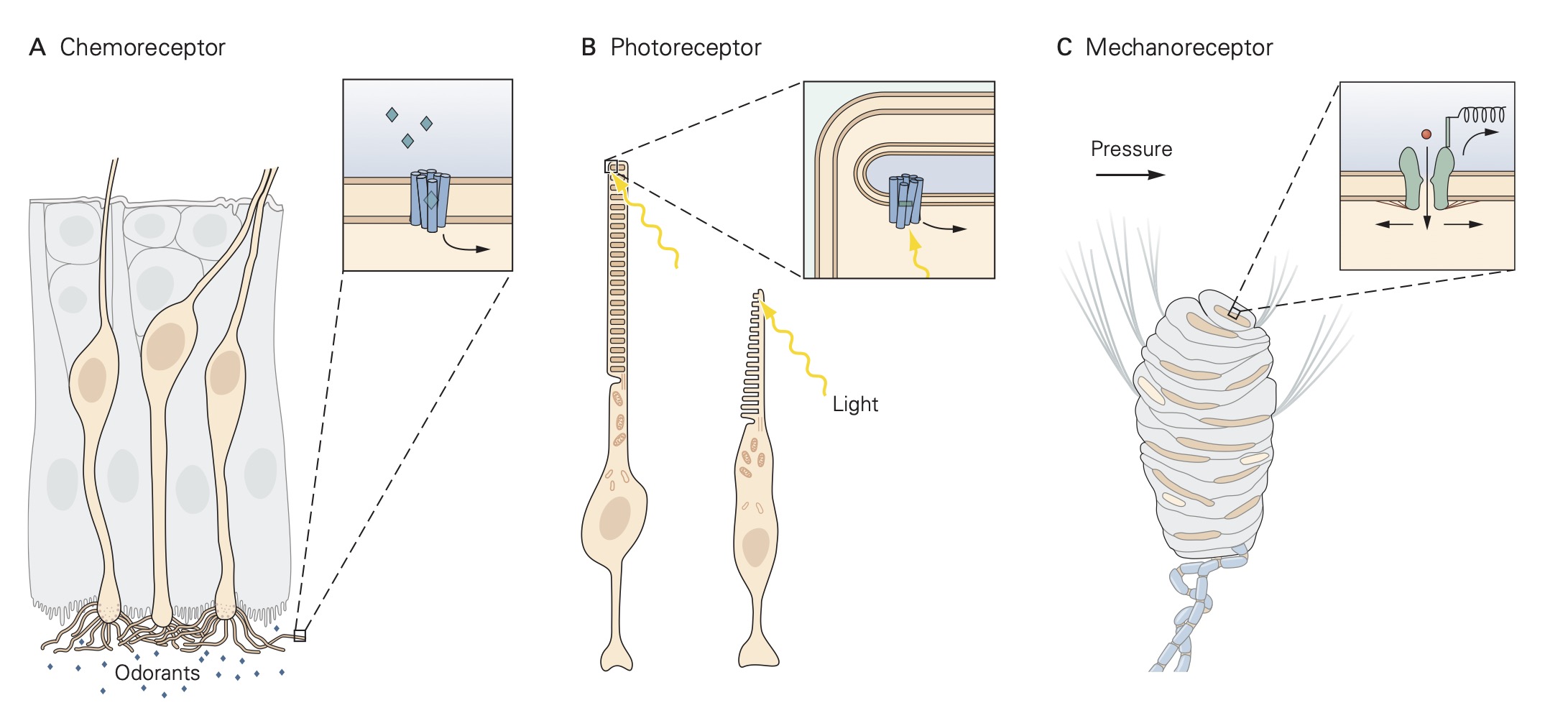
Sensory receptors can be grouped into one of three classes: free nerve endings, nerve endings surrounded by accessory structures, and specialized sensory receptor cells . For free nerve ending receptors, the stimulus interacts directly with the receptor proteins in the sensory receptor’s membrane causing a localized depolarization called a receptor potential. This depolarization spreads electronically along the sensory receptor’s axon where it encounters voltage-gated sodium and potassium channels that generate action potentials. The action potentials are propagated along the axon until they reach the central terminals of the sensory neuron within the central nervous system (CNS) where they initiate the release of neurotransmitters at synapses that excite other nerve cells, called secondary sensory neurons. These secondary sensory neurons carry the signal from the sensory receptors to various regions of the CNS.
The sequence of events described above is the same for sensory receptors with accessory structures except that the stimulus interacts directly with the accessory structure rather than the receptor proteins directly. The accessory structure shapes or filters the original stimulus before in engages with the receptor proteins. For example, the cornea and lens of the eye are accessory structures that serve to focus incident light that enters the pupil onto the rod and cone sensory receptors in the retina located at the back of the eye. The third class of sensory receptors, specialized receptor cells, lacks an axon and thus, do not generate action potentials. When specialized receptor cells interact with their adequate stimulus, a change in membrane potential occurs which leads to either an increase or a decrease in the amount of neurotransmitter that they release onto post-synaptic sensory nerve cells.
Learning Objective #2. To understand the labeled-line principle of signal detection.
Sensory receptors typically respond to only one type of stimulus and that behavior confers selectivity and specificity to the receptor. The stimulus specificity of the receptor is conserved as the signal is propagated along neural pathways in the CNS. The series of neurons that are connected (by synapses) from the sensory receptor to secondary sensory and third order sensory neurons in the CNS is referred to as a ‘labeled line’. The figure below illustrates how the labeled lines for several different sensory modalities are arranged. Because each neuron in the labeled line receives synaptic input from only one type of sensory neuron, each cell in the sequence or circuit retains the identity of the stimulus. The labeled lines enable the brain to distinguish and process information about different types physical stimuli.
Verification of the labeled line principle comes from a variety of experiments performed on human subjects both in laboratory settings and during neurosurgical procedures. One can record the action potentials generated by individual sensory neurons by inserting small wire electrodes into a peripheral nerve in the arm or leg. By probing the cutaneous surface, the experimenter can locate the area of skin that is innervated by the sensory neuron, referred to as the receptive field. Typically, pressing on that area increases the rate of action potentials produced by the sensory neuron. If the experimenter then delivers a series of minute electrical shocks through the same set of wire electrodes, the subject will experience the same sensation as that produced by the pressure. Moreover, the subject will be able to localize the sensation to the receptive field of the sensory neuron whose axon is being stimulated. Similarly, during a neurosurgical procedure to implant a brain stimulator, if the electrophysiologist on the surgical team delivers a small electrical shock through the brain probe near the optic nerve, the awake patient will report that they “saw” a flash of light. If the shock were delivered near the auditory nerve, the patient would report that they “heard” sound. The fact that the brain correctly associates the electrical impulses in a sensory neuron with the type of physical stimulus that normally activates the neuron forms the basis of ‘neural prostheses’ like cochlear implants to restore hearing.
Learning Objective #3: To compare the mechanisms of sensory transduction in different types of sensory receptors.
All of the tissues in our body are endowed with sensory receptors with the ironic exception of the brain. In the following section, the mechanisms by which several different types of sensory receptors transform a physical stimulus into an electrical signal (i.e., sensory transduction) will be compared.
Light Receptors (Vision)
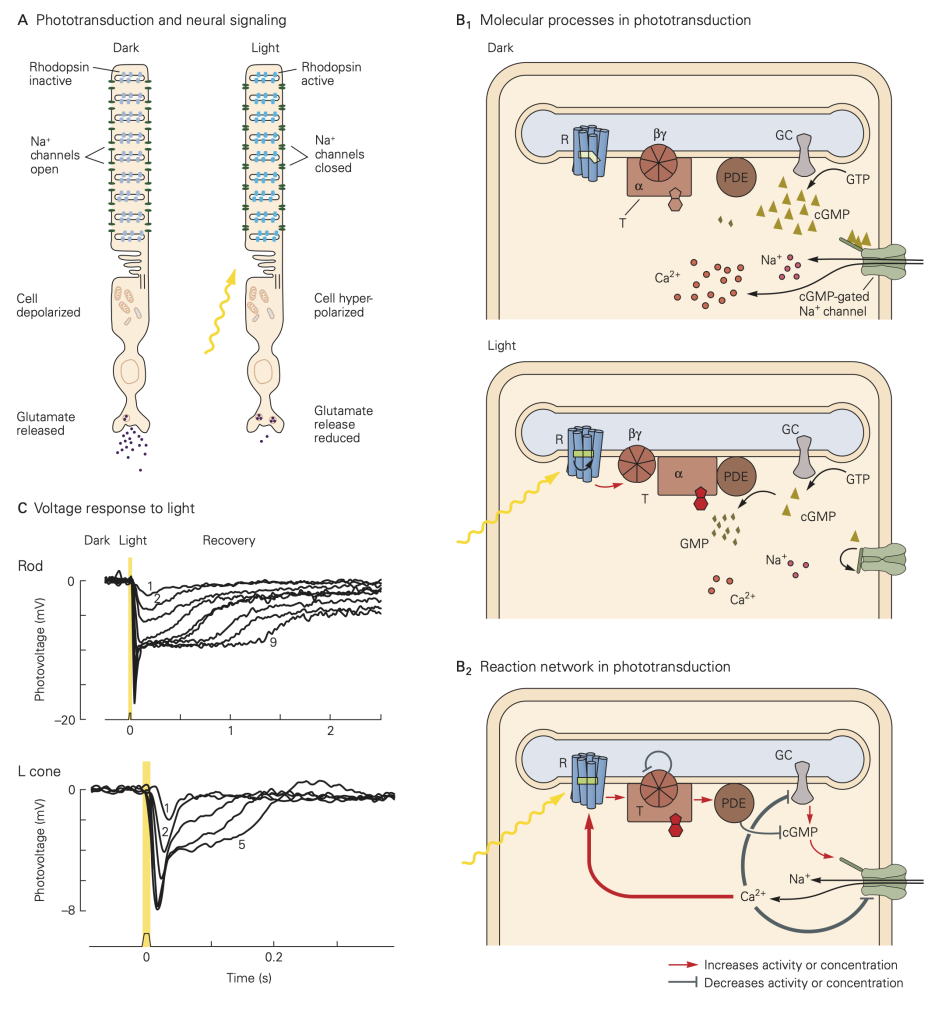
The principal light receptors (i.e., photoreceptors) in humans, rods and cones, are located in a thin layer of tissue lining the back of the eye called the retina. The mechanisms by which the photoreceptors convert light energy to an electrical signal are shown in the figure below. In the absence of a light stimulus, a ‘dark current’ flows through the membrane of the receptors carried by an influx of Na+ ions in the outer segment, and an efflux of K+ ions through the membranes of the inner segments. This current depolarizes the receptor membrane in the dark to about -40 mV. At this membrane potential, Ca2+ ions flow into the synaptic terminals of the photoreceptors resulting in the release of neurotransmitter. When light enters the eye through the pupil and impinges on the retina, it activates as a complex G-protein coupled receptor protein (called a photopigment) embedded on membranous disks within the outer segment of the photoreceptor. The photopigments are composed of a transmembrane opsin moiety and retinal, a chromophore. Absorption of light by the chromophore activates the opsin and then transducin, a G-protein, resulting in a decrease in the concentration of cGMP through phosphodiesterase (PDE) in the cytosol. The decrease in cGMP concentration in turns allows some of the Na+ channels to close, reducing the dark current, hyperpolarizing the receptor, reducing the influx Ca2+ in the synaptic terminals and consequently deceasing the release of neurotransmitter. This sequence of events is referred to as the phototransduction cascade. The process is terminated by the phosphorylation of the photopigment by a kinase and the binding of another protein called arrestin.
Chemoreceptors (Smell and Taste)
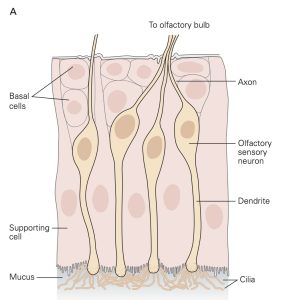
Sensory receptors that are designed to detect the presence of chemicals in the air we breathe, the substances we ingest and the fluids that circulate through our tissues are called chemoreceptors. The most familiar of these are olfactory sensory neurons (OSNs) embedded in the olfactory mucosa and the taste receptor cells found in the taste buds on the tongue and elsewhere in the oral cavity. The OSNs are endowed with olfactory cilia that extend into the mucosa of the oral cavity and interact with airborne odorant molecules. The olfactory cilia of each OSN express only one of the approximately 400 different olfactory receptor G-proteins such that each OSN is activated by a restricted set of odorant molecules. The olfactory sensory transduction cascade is quite similar to the phototransduction cascade except that the G-protein interacts with cyclase to increase cAMP rather than cGMP. The resultant depolarizing receptor potential leads to the generation of action potentials along the axon of the OSN. As is the case in phototransduction, the OSN receptor potential lasts until the olfactory receptor protein is phosphorylated by a kinase and bound to an arrestin-like protein so that the cAMP that was produced is hydrolyzed by PDE.
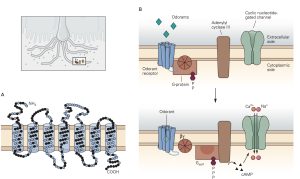
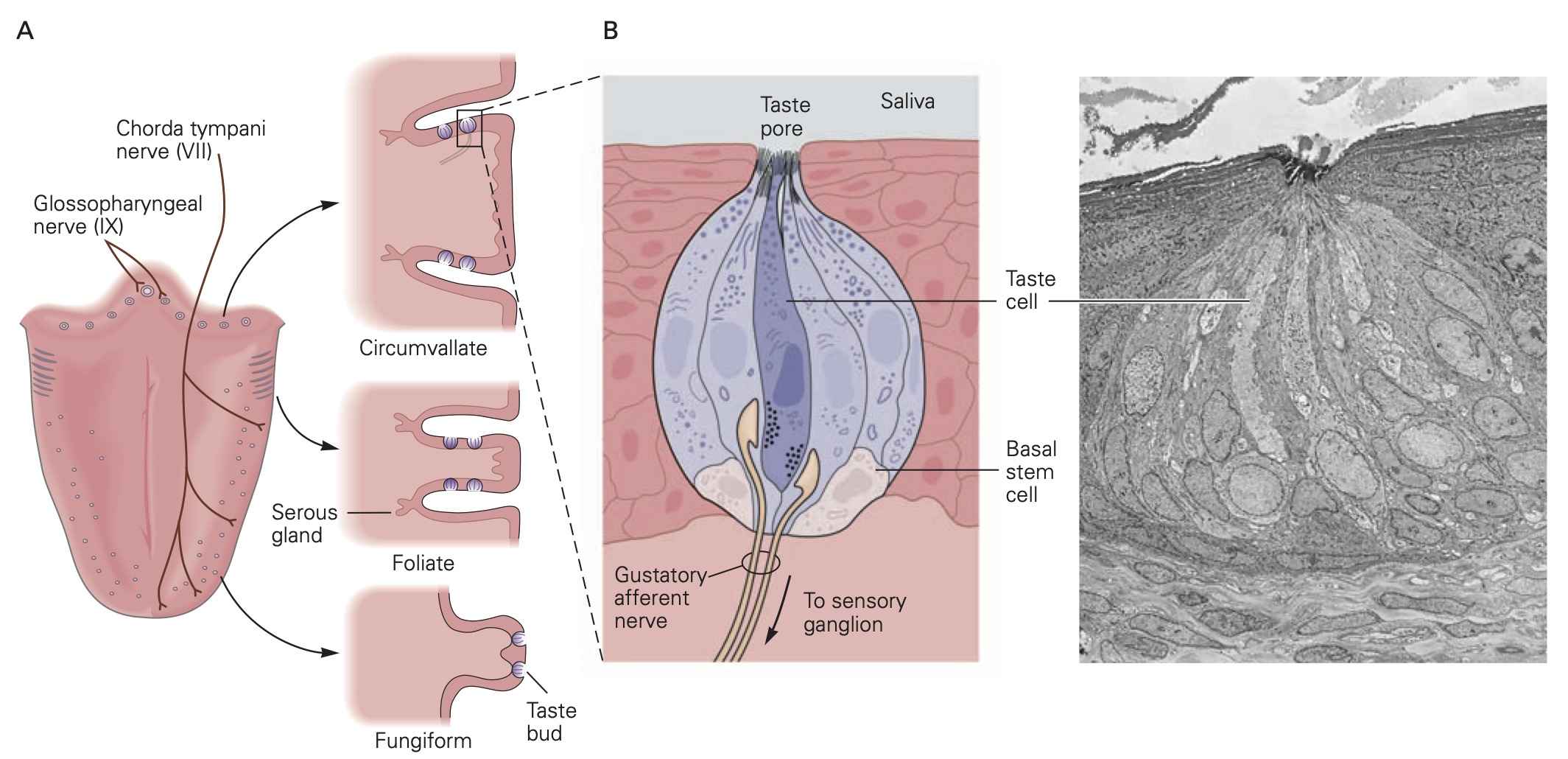
There are several different types of taste cells found in individual taste buds and three different types of sensory transduction mechanisms. Tastant molecules contained in ingested materials enter the taste buds through a taste pore on the surface of the tongue and other oral cavity structures and interact with taste receptors located on the microvilli of the taste cells. Full elucidation of the different transduction mechanisms underlying taste remains elusive, but the following descriptions are reasonable secure. Sweet, bitter and savory (umami) molecules bind to G-protein coupled taste receptors called, gustducins, leading to a depolarizing receptor potential in the taste that increases the release of neurotransmitter from the distal synaptic terminals. Sour, acidic substances and CO2 produce H+ ions in solutions that bind to sour taste receptor proteins and also flow through non-specific cation channels in the microvilli of sour taste cells. Finally, edible salts dissociate in liquid and the Na+ ions enter salty taste cells through ion channels in their microvilli leading to depolarization.
Mechanoreceptors (Touch and Proprioception)
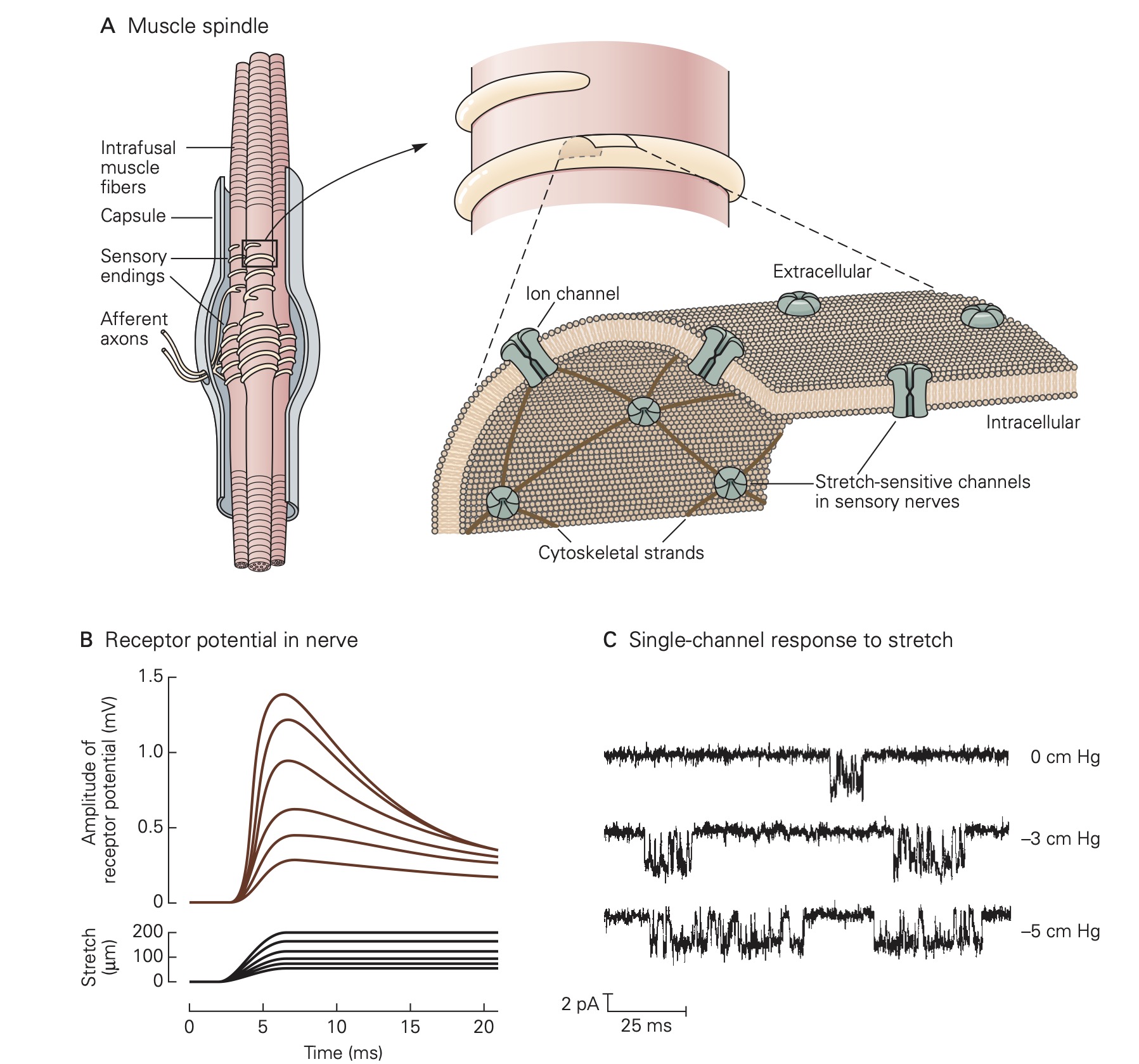
Virtually of the tissues in the body are endowed with some type of mechanoreceptor designed to detect physical displacement, i.e., movement. For example, blood vessels are innervated by baroreceptors that respond to stretch of the vessel wall and thereby signal blood pressure to the CNS. Skeletal muscles contain two types of proprioceptors, called muscle spindles and Golgi tendon organs, which transduce muscle length and muscle force, respectively, into electrical signals that permit the CNS to precisely control movement. Common to all mechanoreceptors are membrane receptor proteins that form ion channels whose gating depends mechanical forces exerted on the channel protein. For example, applying stretch to a muscle also stretches the muscle spindles that reside within the muscle. This causes the stretch-sensitive channels in the membrane of the muscle spindle sensory receptors to open allowing Na+ ions to flow into the sensory neuron which in turn results in a depolarizing receptor potential. Relaxing the muscle stretch, removes the force on the mechanosensitive channels on the muscle spindle sensory receptors and the membrane of the sensory neuron returns to its resting level.
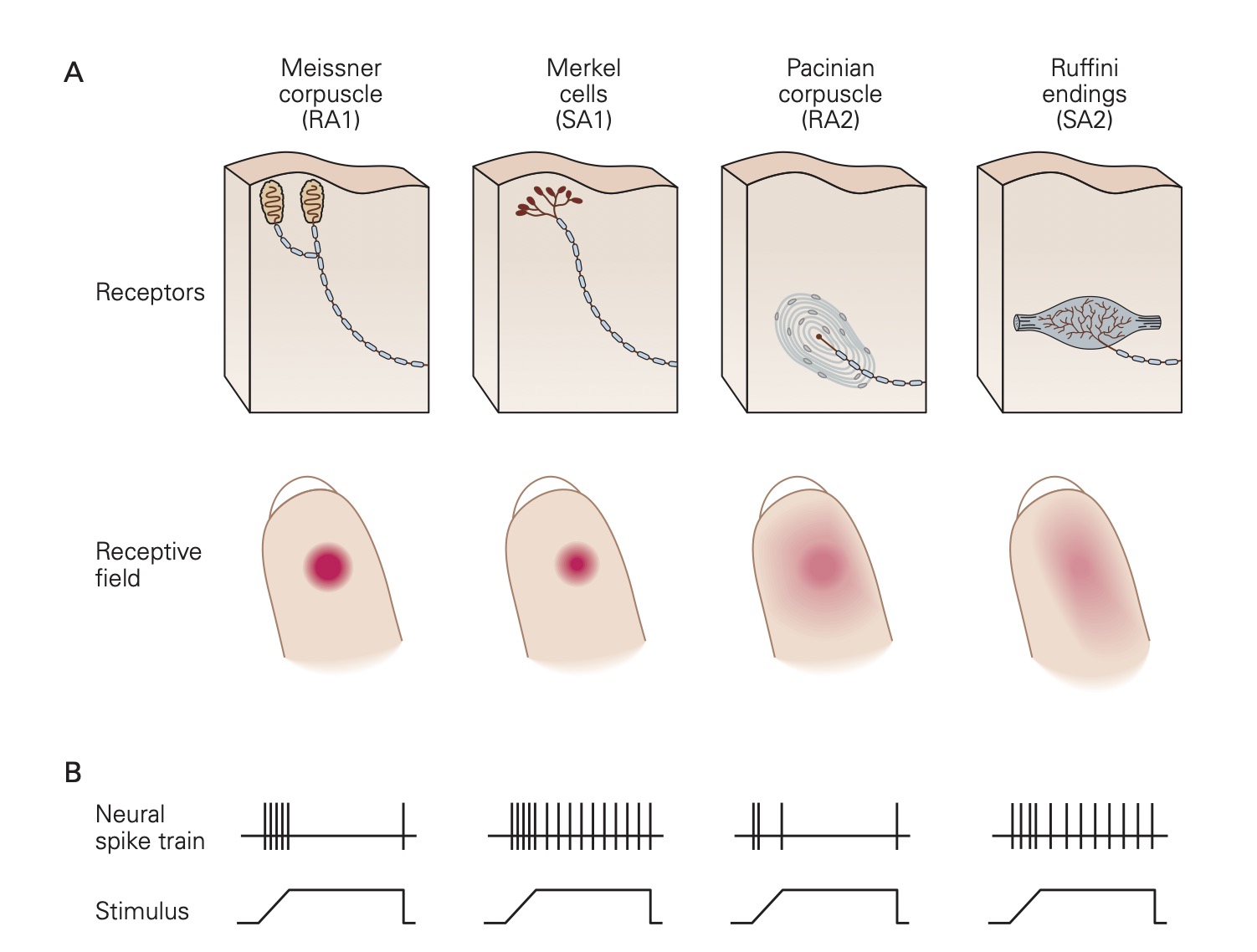
Skin is richly endowed with a host of different types of mechanoreceptors; each specialized to detect a particular form of mechanical stimulation. Also found in skin and many other tissues are nociceptors that are designed to detect noxious stimuli like extreme pressure, extreme temperature and dangerously high levels of chemical substances.
Hair Cell Receptors (Hearing and Balance)
A highly specialized form of mechanoreceptor called the hair cell is found in the four different sense organs that reside within the bony labyrinth (i.e., cochlea, semicircular canals, utricle and saccule). The hair cells in the cochlea transduce sound waves that impinge on the tympanic membrane into changes in membrane potential and the hair cells in the semicircular canals, utricle and saccule are activated by movements of the head and body to help maintain object fixation in the visual field and balance.
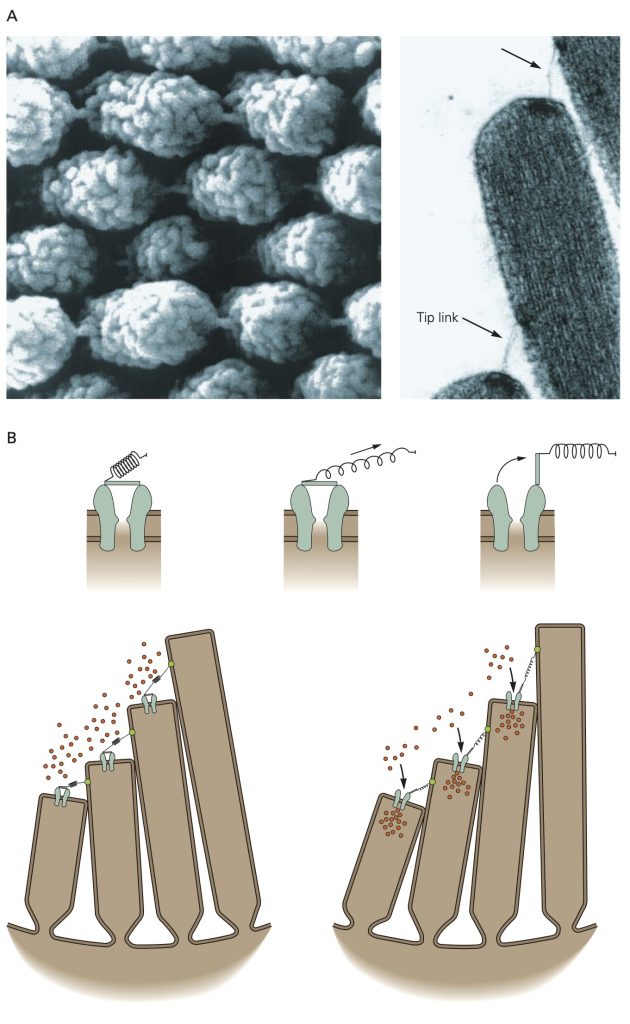
Hair cells reside in an unusual extracellular milieu: the apical end that include the stereocilia that give the hair cells their name projects into endolymph which has an ionic composition similar cytosol (i.e., high K+, low Na+), while the remainder of the cell is bathed in perilymph with an ionic concentration similar to other extracellular fluids (high Na+, low K+). Pump molecules in the adjacent epithelia cells maintain the disparate ionic concentrations in these two compartments of the hair cells. K+ ions flow through non-selective cation channels located in the tips of the stereocilia, some of which are open at rest. Displacement of the stereocilia either stretches or relaxes molecular linkages between adjacent stereocilia that opens or closes the K+ channels, respectively. Opening more K+ channels leads to a depolarizing receptor potential whereas closing the K+ channels leads to a hyperpolarizing receptor potential. Depolarizing receptor potentials activate voltage-gated Ca2+ channels in the hair cell that results in more neurotransmitter release. With hyperpolarizing receptor potentials, the Ca2+ channels remain closed and the amount of neurotransmitter released is decreased.
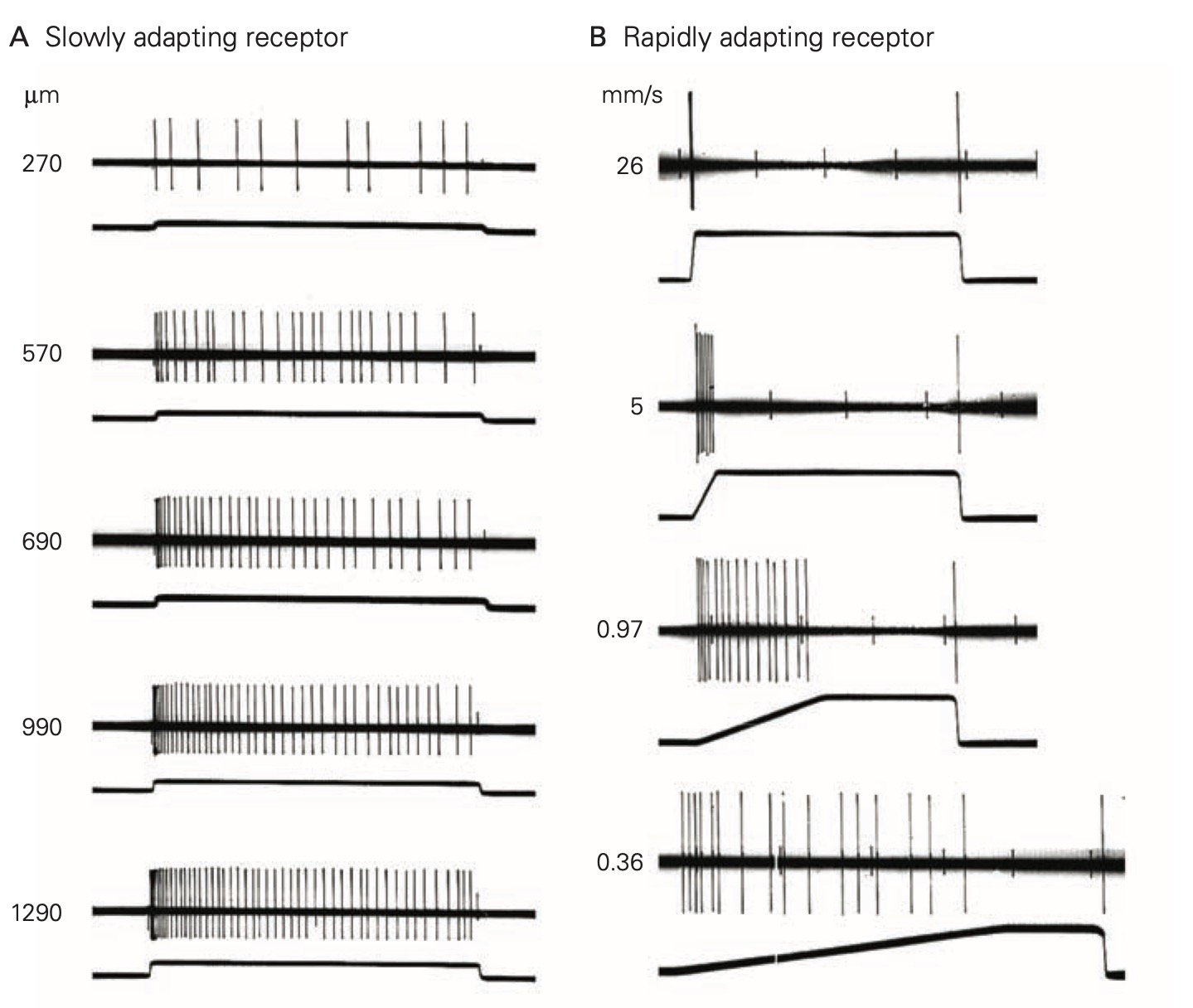
Unlike the all-or-none action potential, receptor potentials are graded and are proportional to the strength of the stimulus that evokes them. Further, receptor potentials normally do not display refractoriness: They persist as long as the stimulus is present. The magnitude of the receptor potential is then reflected in either the rate or action potential generation (i.e., discharge or firing rate) in the sensory neuron or in the amount of neurotransmitter released in a specialized sensory receptor cell that lack axons. The relationship between the magnitude of the receptor potential and the firing rate of the sensory neuron (or the amount of neurotransmitter released for a specialized receptor cell that does not produce action potentials) forms the basis of the neural code, the firing rate or amount of transmitter released ‘reports’ the strength of a stimulus.
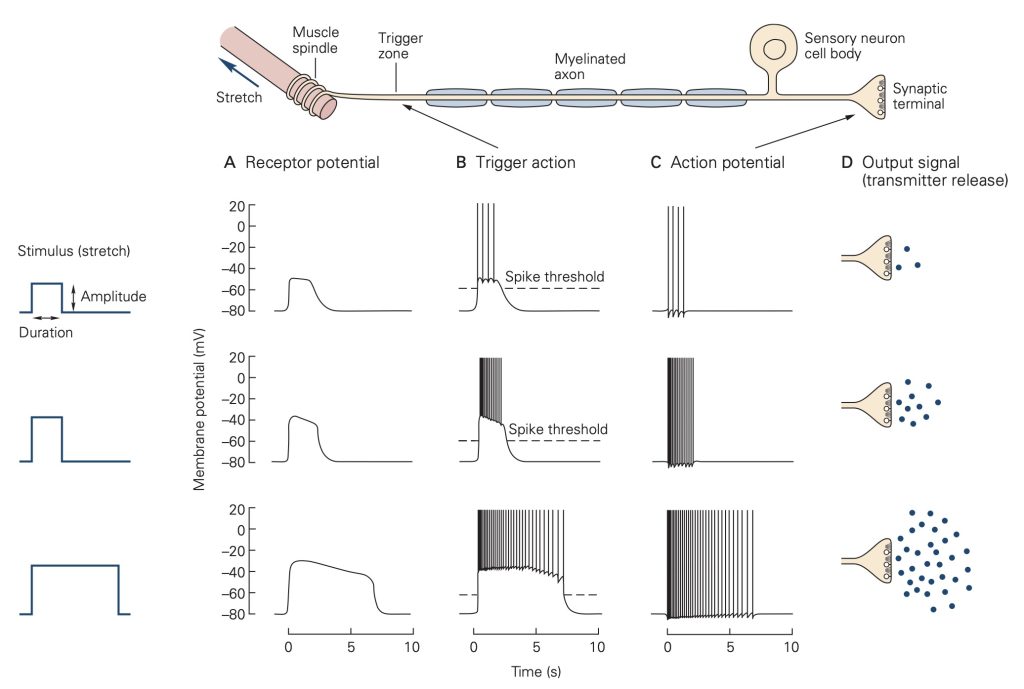
In response to a constant stimulus, most sensory receptors do not maintain a constant receptor potential, but rather one that declines over time. This process is called adaptation and is mediated by several different mechanisms in different receptors including changes in the behavior of the accessory structures, intracellular signal cascades and even changes in the threshold for generating action potentials at the axon hillock of the sensory neuron. Some sensory receptors adapt slowly to a constant stimulus and others adapt more rapidly. Slowly-adapting receptors are sometimes called tonic receptors because their sensory neurons maintain a tonic or sustained level of discharge as long as the stimulus is present. Likewise, rapidly-adapting sensory receptors are referred to as phasic receptors because they only discharge during specific phases of the stimulus, normally at the onset, but sometimes also at the offset. Tonic receptors are designed to signal the continued presence of a stimulus, whereas phasic receptors primarily signal the start time and end time of the stimulus. Many tissues, like skin and sense organs, like muscle spindles are innervated by both tonic and phasic receptors.