22 Regulation of Water Balance
Water Reabsorption
Water reabsorption is a passive process: water is reabsorbed by osmosis. Recall that osmosis is the movement of water across a water-permeable membrane towards a region of higher solute concentration.
In most of the nephron there is unregulated isosmotic reabsorption of water and solute, in other words, water reabsorption is coupled to solute reabsorption. However, it is possible to reabsorb water independently of solute to produce a concentrated urine, that is, urine that has a higher osmolarity than the extracellular fluid.
Regulated water reabsorption occurs at the medullary collecting duct. The figure below is a schematic showing the last part of a nephron. The ability to excrete urine that is more concentrated than the extracellular fluid (ECF) depends upon the loops of Henle, which function to concentrate osmolarity in the deepest part of the medulla, creating a vertical osmotic gradient. As the collecting duct descends through the medulla, the increasing osmolarity in the surrounding interstitial fluid drives water reabsorption.
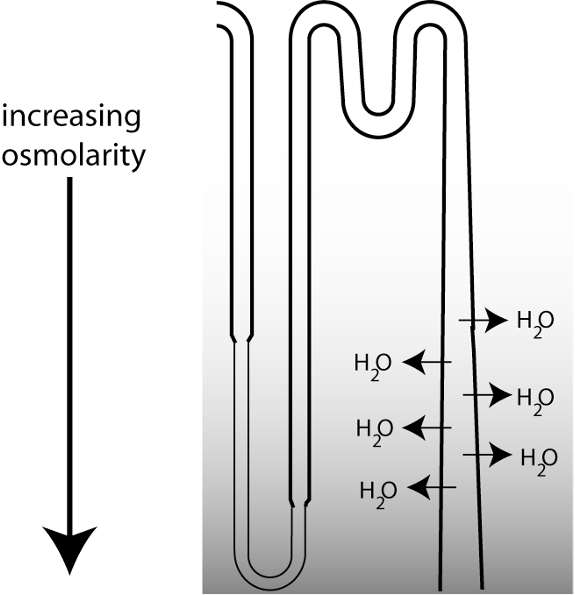
However, there is a tremendous variability in water excretion. The urine produced can either be concentrated, or very dilute. How do the kidneys vary their urine concentrating ability? They do so by regulating water permeability in the collecting duct.
Regulated Permeability in the Collecting Duct
In humans, the vertical osmotic gradient in the medulla allows the kidneys to produce urine that can be roughly 5 times as concentrated as the ECF. Urine concentration can be varied through the regulation of water permeability in the collecting duct. The figure below reminds you of the histology of the collecting duct.
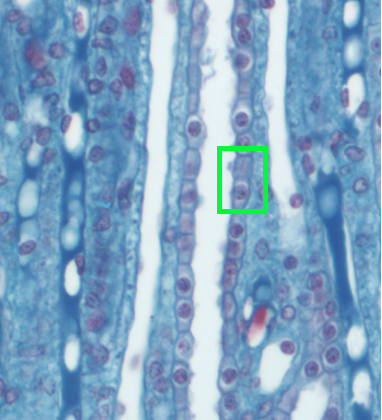
The permeability of cell membranes to water depends upon the presence of water channels known as aquaporins. There is a family of aquaporin proteins, with different types being expressed in different tissues.
The figure below is an illustration of epithelial cells in the medullary collecting duct.
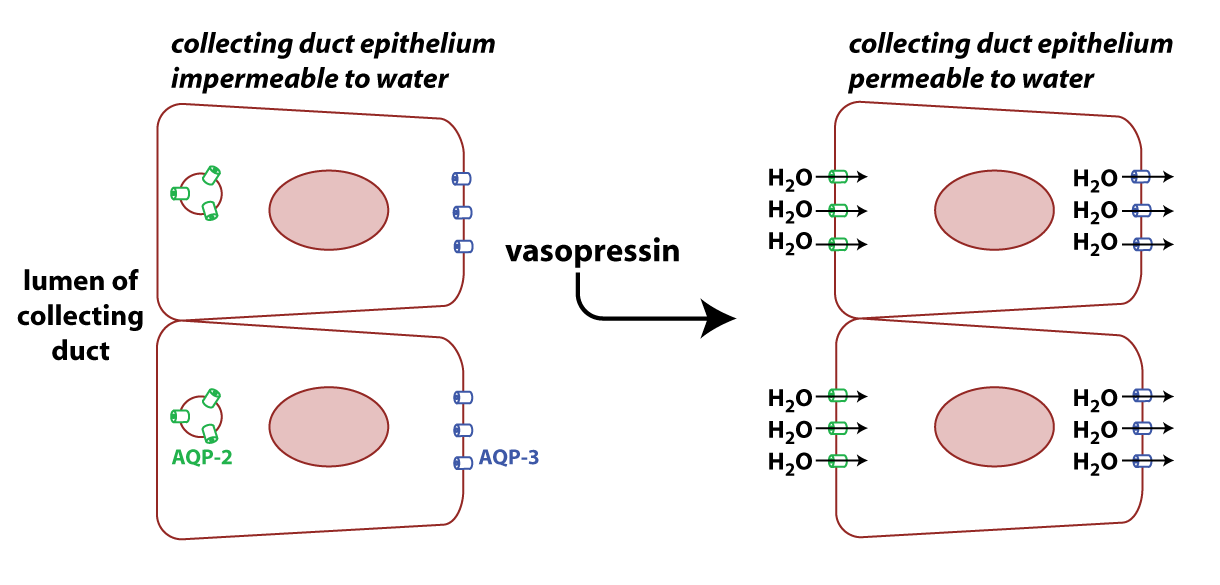
AQP3 (blue in figure) is constitutively expressed on the basolateral surface of cells in the collecting duct. AQP2 (green in the figure) is found on the apical surface of these cells, but the number of AQP2 channels on the membrane is regulated by the peptide hormone vasopressin (also known as antidiuretic hormone). Note that clinicians refer to the human form of vasopressin as “arginine vasopressin” or AVP because it has an arginine in the eighth position of the peptide.
When vasopressin binds to its receptor on the collecting duct cells, it stimulates the translocation of AQP2 to the membrane by causing vesicles containing the protein to fuse with the plasma membrane. The result is more AQP2 proteins on the apical membrane and higher permeability to water.
Regulation of Vasopressin Secretion
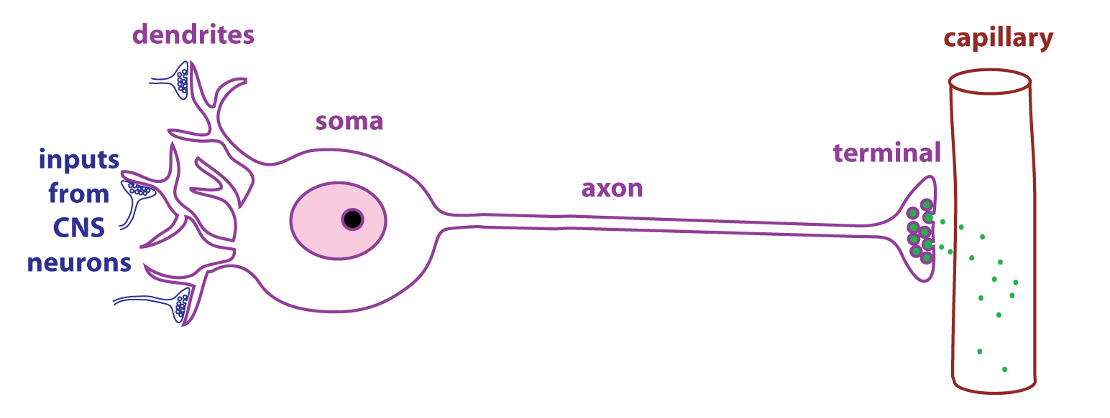
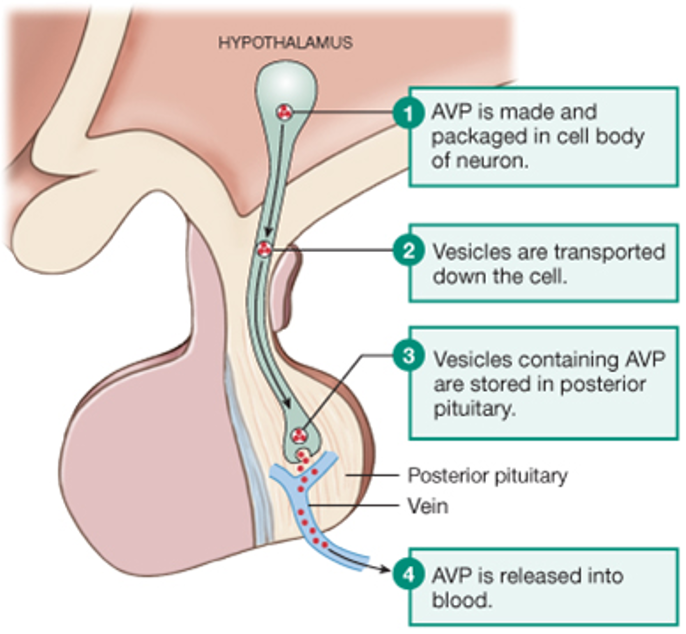
Vasopressin is a peptide hormone that is produced by neurosecretory cells, a type of endocrine cell found in the hypothalamus of the brain. As shown in the figure, neurosecretory cells have dendrites, axons, and terminals just like typical neurons. The difference is that the terminals of neurosecretory cells are adjacent to capillaries. Neurosecretory cells secrete regulatory molecules (green dots) that enter the circulation and act as hormones.
Vasopressin is secreted from the posterior pituitary. The pituitary gland sits below the hypothalamus and consists of two parts.
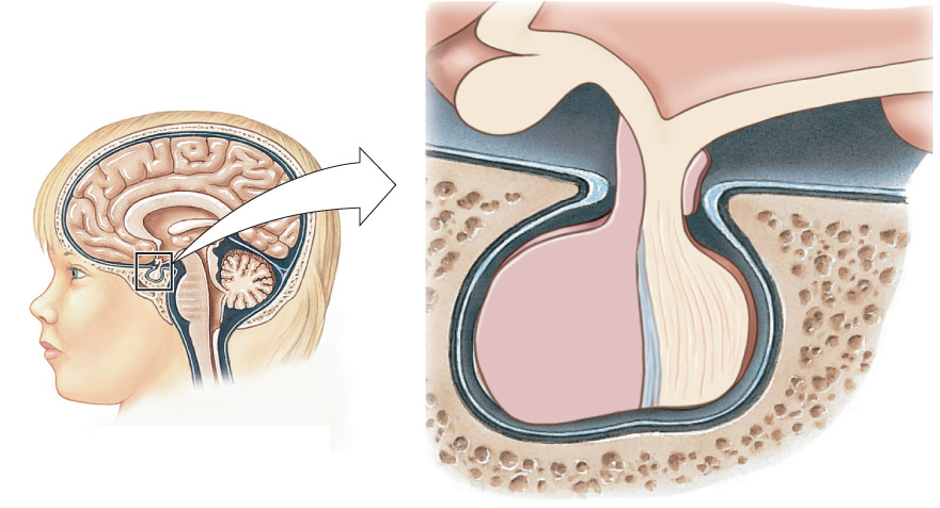
The anterior pituitary (or adenohypophysis) is the glandular part of the pituitary and contains endocrine cells releasing a variety of hormones that control growth, reproduction, lactation, thyroid function, and adrenal function. The posterior pituitary (or neurohypophysis) is effectively an extension of the hypothalamus and contains the axons and terminals of large neurosecretory cells whose cell bodies are found in the hypothalamus. The figure below schematizes how a large neurosecretory cell secretes vasopressin at the posterior pituitary.
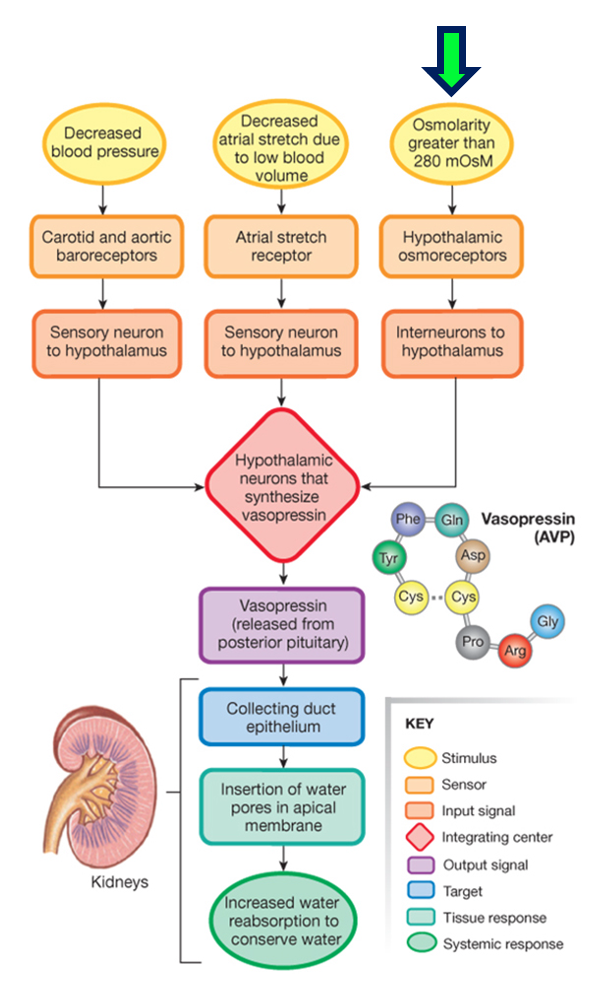
The figure below details all the ways that vasopressin secretion can be triggered.
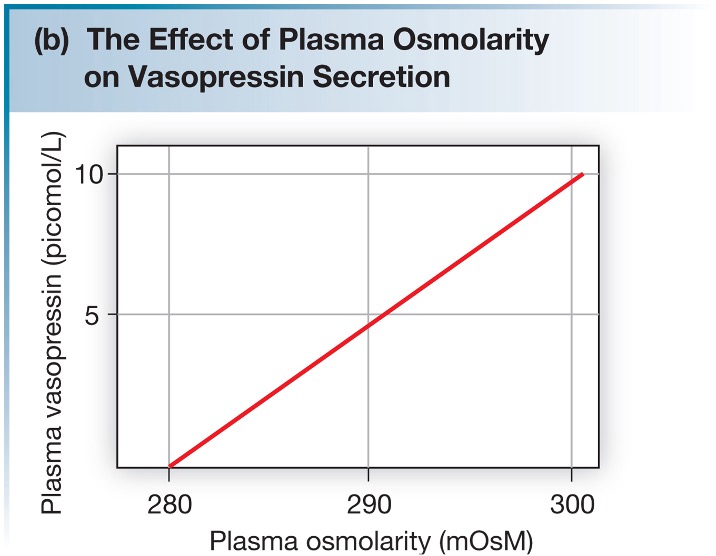
The primary way that vasopressin secretion is stimulated is through changes in osmolarity. As shown in the graph below, there is a linear relationship between plasma vasopressin levels and ECF osmolarity.
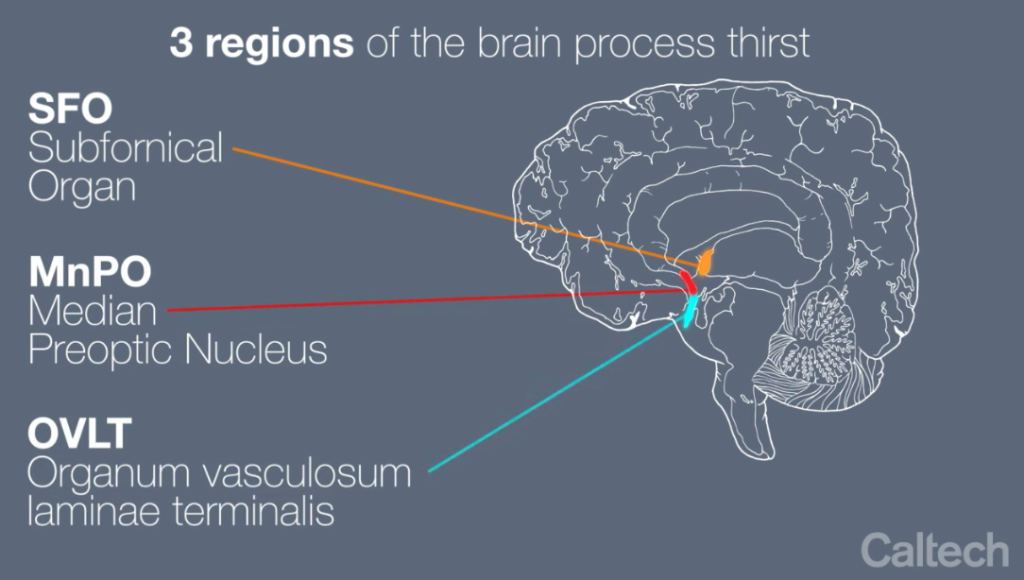
The changes in ECF osmolarity that stimulate vasopressin secretion are detected by hypothalamic neurons called osmoreceptors. If the osmolarity of the ECF increases, the osmoreceptors increase their frequency of action potential firing, and more vasopressin is secreted. Increased action potential firing by the osmoreceptors also stimulates thirst. If the osmolarity of the ECF decreases, the osmoreceptors decrease their action potential frequency and less vasopressin is secreted. The osmoreceptors are located in two nuclei that are adjacent to the third ventricle and outside the blood-brain barrier. These nuclei are called the organum vasculosum of the laminate terminalis (OVLT) and the subfornical organ (SFO). The figure below shows the relative location of these structures in the brain.
AVP-D and AVP-R are Disorders in the Ability to Concentrate Urine
If there is a problem with vasopressin action, the result is an inability to concentrate urine, which leads to polyuria (a high urine volume). This can be caused by a lack of vasopressin (arginine vasopressin deficiency* or AVP-D; formerly known as central diabetes insipidus) or due to a defect in the ability of the kidney to respond to vasopressin (arginine vasopressin resistance or AVP-R; formerly known as nephrogenic diabetes insipidus). AVP-D may be caused by a genetic mutation where vasopressin is missing or defective. Head trauma, a tumor, or injury to the posterior pituitary may also cause AVP-D. AVP-D is treated with desmopressin, a synthetic vasopressin agonist.
AVP-R can be caused by two types of congenital defect. Mutations in the vasopressin receptor cause AVP-R. Another type of mutation that causes the disorder involves a defect in the gene for AQP2. This defect prevents the proper localization of AQP2 proteins on the apical membrane of collecting duct cells. Acquired AVP-R is not due to a genetic defect, and therefore not present from birth like a congenital defect. The drug lithium, which is used in the treatment of bipolar disorder, can cause acquired AVP-R. An important characteristic of AVP-R is that it doesn’t respond to treatment with desmopressin, since the defect is in the kidney’s response to the hormone. AVP-R is treated with supportive therapy.
Summary: Homeostatic Regulation of ECF Osmolarity
The figure illustrates the regulation of water balance as a negative feedback regulatory system, showing what occurs when there is loss of water through sweating to cause a temporary increase in extracellular fluid osmolarity.
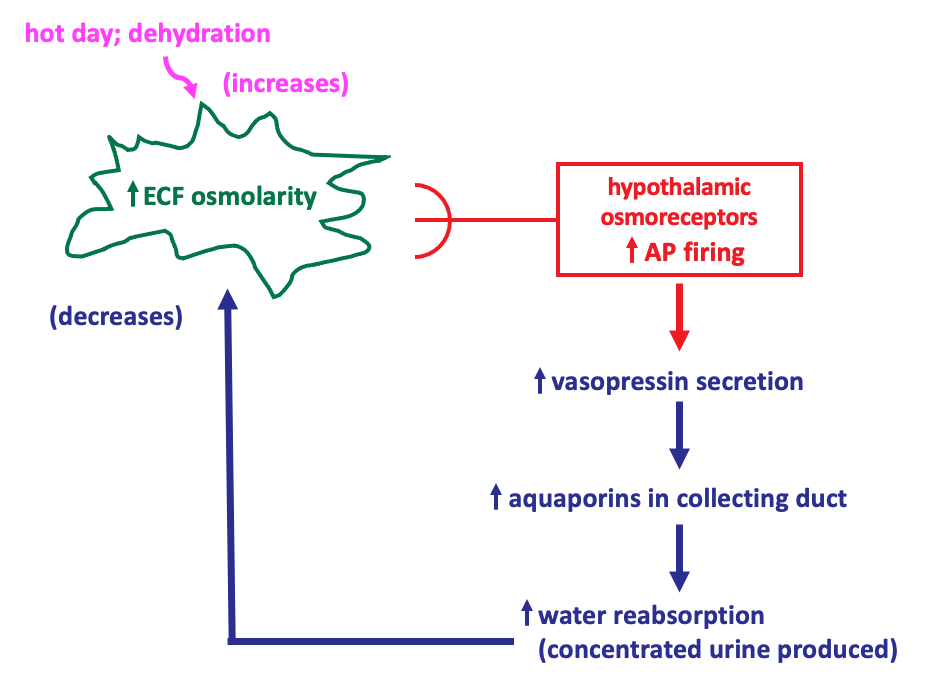
The regulated variable is the ECF osmolarity. The sensors are the hypothalamic osmoreceptors, which modulate their frequency of action potential firing in response to changes in ECF osmolarity. The effector system that restores ECF osmolarity to its set point involves vasopressin and its effects on water reabsorption in the collecting duct.
