21 Polyuria in Diabetes Mellitus
Introduction
Diabetes comes from the Greek word which means “siphon”. In older terminology there were two distinct disorders sharing the first name diabetes: diabetes mellitus and diabetes insipidus. This is because both disorders can cause polyuria, or excessive urine output. Diabetes insipidus is a disorder of urine concentration. To prevent confusion, diabetes insipidus is now called either arginine vasopressin deficiency (AVP-D) or arginine vasopressin resistance (AVP-R). We will the examine the process of urine concentration and these disorders in the next chapter.
Diabetes mellitus is a disorder of blood glucose regulation, which results from a deficiency in the action of the hormone insulin. This may be due to autoimmune destruction of the insulin-secreting cells of the pancreas (type 1 diabetes mellitus) or it may result from a problem in the responsiveness of tissues to insulin, known as insulin resistance (type 2 diabetes mellitus). With either disorder, the result is hyperglycemia, or high levels of glucose in the plasma. In many cases hyperglycemia does not cause any symptoms, but in some individuals it causes excessive urination and increased thirst.
Basis for Polyuria
How does hyperglycemia cause excessive urine production? To answer this, we need to understand a little bit about how the kidney works. Each kidney contains about a million functional units called nephrons (blue structure in the figure below).
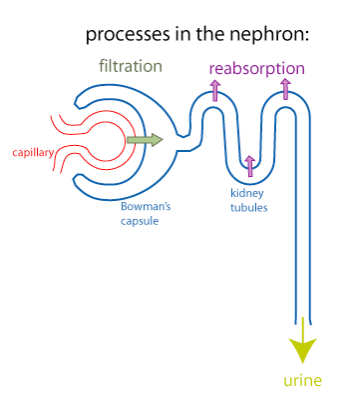
The first step in the production of urine is a process called filtration (green arrow). In filtration, there is bulk flow of water and small molecules from the plasma into Bowman’s capsule (the first part of the nephron). Because of the nonspecific nature of filtration, useful small molecules such as glucose, amino acids, and certain ions end up in the forming urine, which flows into the kidney tubules. To prevent the loss of these useful substances from the body, the cells of the kidney tubules use epithelial transport to transfer these substances out of the forming urine and back into the extracellular fluid. This process is known as reabsorption (purple arrows). Reabsorption of glucose specifically occurs in the proximal tubule. The sodium-glucose cotransporter that is specific to the kidney tubules is called SGLT2.
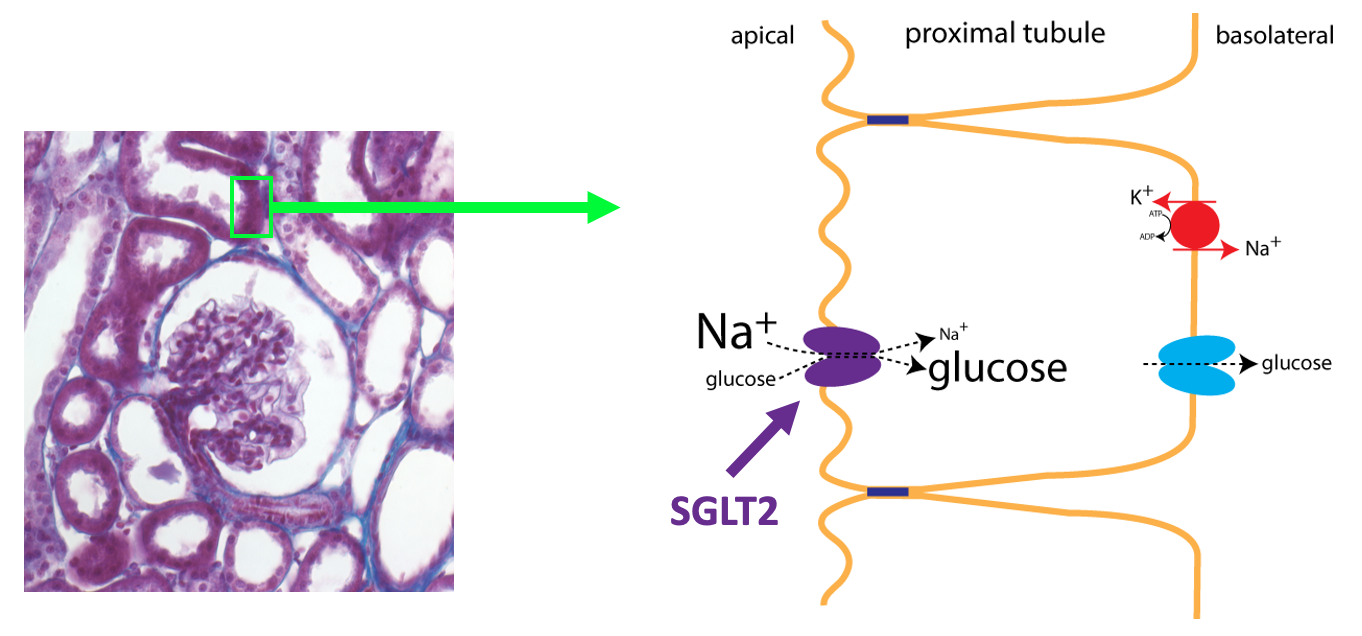
Under normal circumstances, 100% of the glucose that is filtered is reabsorbed. Glucose reabsorption in the proximal tubule utilizes the same basic process as glucose absorption in the small intestine as outlined in the figure below (see also the chapter on Epithelial Transport).
Because filtration involves bulk flow, as the concentration of a substance increases in the plasma, the filtered load (amount filtered) will also increase, as shown in the lefthand graph in the figure below.
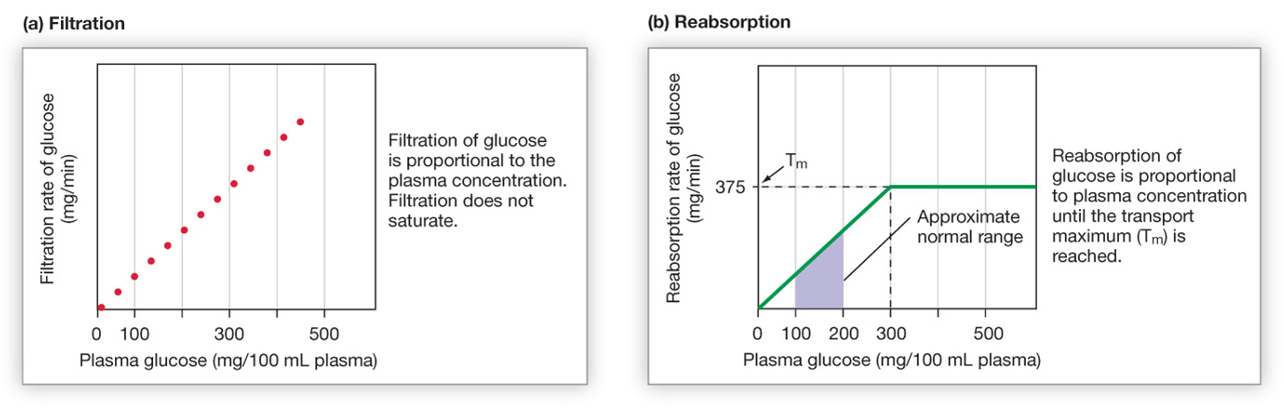
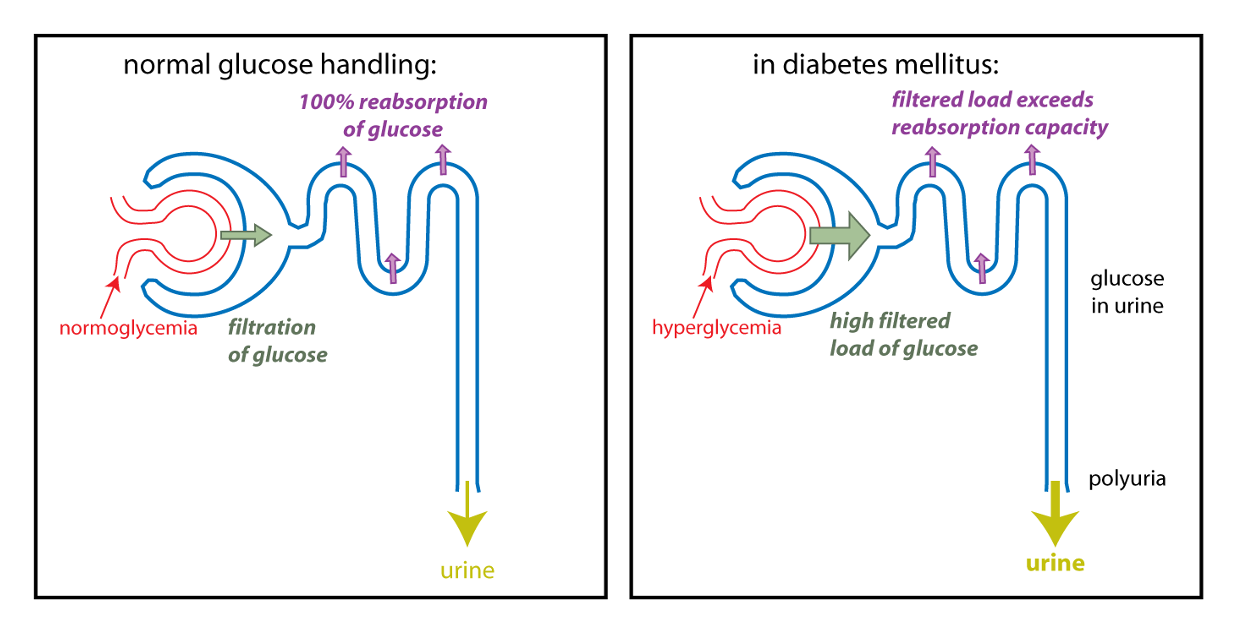
As shown on the right, the rate of reabsorption reaches a maximum when all the binding sites on SGLT2 become occupied (saturation). In a diabetic that has hyperglycemia, the filtered load of glucose (amount of glucose filtered) can exceed the capacity of the kidney tubules to reabsorb glucose, because the transport proteins become saturated. Once the maximal rate of transport is reached, some glucose in the filtrate will NOT be reabsorbed. The result is glucose in the urine. Glucose is a solute that draws water into the urine by osmosis. Thus, hyperglycemia can cause a diabetic to produce a high volume of glucose-containing urine, in other words, polyuria.
SGLT2 Inhibitors
About 90% of renal glucose reabsorption occurs via SGLT2. SGLT2 inhibitors are drugs that inhibit glucose reabsorption by competing with glucose for binding to the SGLT2 protein. They were initially designed to treat diabetes mellitus by causing glucose loss in the urine as a way to combat hyperglycemia. The first SGLT2 inhibitor was approved in 2013. There are now five FDA-approved SGLT2 inhibitors on the market[1]. In drug trials involving diabetic patients, these drugs have been shown to reduce hyperglycemia, promote weight loss, and cause a small reduction in blood pressure. The small amount of weight loss, along with the fact that SGLT2 inhibitors are oral drugs (taken as a pill and not as an injection) make them appealing drugs for the treatment of type 2 diabetes, which is usually associated with overweight and obesity.
SGLT2 inhibitors have become very important drugs, not only in the treatment of type 2 diabetes mellitus, but also for the treatment of heart failure and kidney disease. Since 2008, companies developing drugs to treat diabetes mellitus have been required to perform additional testing to make sure that the drug does not cause increased risk of cardiovascular disease. Multiple large studies showed that SGLT2 inhibitors were safe, and actually decreased the risk of death from cardiovascular disease and of hospitalization for heart failure. Further studies showed that SGLT2 inhibitors reduced the risk for progression of chronic kidney disease. Preventing or delaying cardiovascular and kidney disease is an important goal since these diseases are major causes of mortality in diabetics.
SGLT2 inhibitors were then tested for the treatment of heart failure and chronic kidney disease in non-diabetics and shown to have a benefit. SGLT2 inhibitors are now FDA approved to treat not only type 2 diabetes mellitus, but also heart failure and chronic kidney disease. The exact mechanisms whereby SGLT2 inhibitors provide benefit in heart and kidney disease are still being worked out.
- SGLT2 inhibitors all have "-gliflozin" as a suffix in their generic name. Canagliflozin (tradename: Invokana) was approved in May 2013. Dapagliflozin (tradename: Farxiga) was approved in January 2014. Empagliflozin (tradename: Jardiance) was approved in August 2014. Ertugliflozin (tradename: Steglatro) was approved in December 2017. Bexagliflozin (tradename: Brenzavvy) was approved in January of 2023. ↵
