1 Principles of Cancer Therapy
Learning Objectives
1. Understand the meaning of adjuvant therapy and related biologic principles
2. Describe how overall performance status and medical comorbidities affect treatment options
3. Learn principles behind new trends in oncologic therapeutics
4. Discuss how access to care and socioeconomic factors impact cancer outcomes
Introduction
Cancer as a local problem
People have been trying to cure cancer through surgery for millennia – with some success. An Egyptian papyrus from 1600 BC describes a procedure to remove breast tumors by cauterization. The Roman physician Celsus (AD 175–177) wrote, “After excision, even when a scar has formed, none the less the disease has returned.” Advances in the 18th and 19th century began to make surgery more feasible and less risky. Germ theory led to antiseptic techniques, and pharmacologic advances allowed general anesthesia. In the early 20th century endotracheal intubation led to controlled airway management, which allowed paralytics to be administered.


One of the most well-known surgeons in history was John Stewart Halsted (1852-1922). Halsted performed the first radical mastectomy in New York, going on to become a prominent surgeon at Johns Hopkins University. In an effort to achieve cure, he performed increasingly aggressive and disfiguring surgeries including resecting chest wall structures including the pectoralis major and minor as well as subclavicular and axillary LNs. He theorized that sufficient removal of tumor and surrounding tissues would ultimately eliminate malignancy – but patients still sometimes recurred. This led to recognition that cancer is a systemic condition, with recurrence and survival more dependent on spread before surgery than tissue removed at surgery.

Cancer as a systemic condition
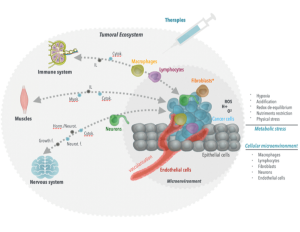
The concept of cancer as a systemic condition is intuitive in hematologic malignancies, i.e., the tumor is in the blood, and blood goes everywhere, so the disease affects the whole organism. But the problem of cancer as a systemic condition runs deeper than the geography of the tumor cells. Cancer uses the body’s own mechanisms to its advantage, like a parasite. A solid tumor starts in a specific spot, but is already harnessing the whole body’s tools to promote its own survival. For example, metabolic stresses such as hypoxia, acidification, and nutrient deprivation drive shifts towards glycolysis and angiogenesis, promoting blood vessel growth to the tumor. Other signaling pathways can lead to hormonal support of the tumor and immune evasion – all creating a tumor “ecosystem” that promotes the growth of cancer – and eventually, as a solid tumor evolves, it gains the ability to colonize the body with malignant cells (see fig below).
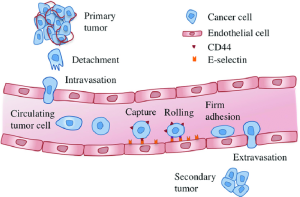
If we remove a tumor early, before systemic colonization occurs, that alone can be curative. Spread, or colonization of the tumor, also known as metastasis, involves movement of tumor cells from primary tumor to distant sites. This is a complex process involving:[1]
Local Invasion
Intravasation (tissue → bloodstream)
Hematogenous survival
Extravasation (bloodstream → tissue)
Colonization
This process is dependent on epithelial-mesenchymal transition (EMT): activation of natural cellular programs, leading to cellular changes that activate invasive and metastatic potential.
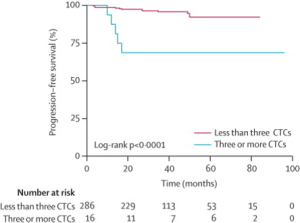
Indeed, so-called circulating tumor cells (CTCs) have been found in the blood of patients with breast, ovarian, colorectal, pancreatic, head and neck cancers, and others. These have been found to be a marker for poor prognosis and risk of recurrence – but detection does not actually guarantee recurrence. CTCs and other methods of detecting so-called “micrometastatic” disease have not reached a point where they can be used to guide therapy. Example of a study of patients with resected breast cancer (stage I-III), where CTCs iden0fied in 25% of patients at surgery, and their presence correlated with worse overall and progression free survival rates, independent of other prognostic factors (sentinel lymph node status, stage, etc.).
Thus, imaging remains the primary means to detect metastasis – but of course imaging is only good for macroscopic tumors. Clinicians and researchers have found empirically that onc metastatic disease sites can be detected on imaging or become symptomatic, this represents a disease that cannot be cured. Many treatments exist to control or mitigate the cancer, but eradication of metastatic malignancy (with a few exceptions) is generally not possible.

Cancer cure: Local control and systemic insurance
If we have no evidence of spread, we assume the tumor is localized, and local therapies that can eradicate that tumor – surgery, radiation – are critical. But we know micrometastases can and often do persist undetected, so treating this invisible disease is also key to cure. This is the rationale for systemic therapy.
Adjuvant/neoadjuvant therapy
Definition of adjuvant
Adjuvant therapy is treatment administered after tumor resection to decrease the risk of recurrence from microscopic residual disease. Adjuvant therapies can be systemic, to protect the patient from metastatic disease, or local, to protect the patient from local recurrence.
Adjuvant therapy can include:
• Chemotherapy
• Hormonal therapy – most commonly for ER/PR+ breast cancer and prostate cancer
• Targeted biologic therapy– for example, use of HER-2 directed therapy trastuzumab in resected HER2+ breast cancer
• Immunotherapy – Increasingly being investigated in clinical trials
• Radiation therapy – often used to decrease risk of local recurrence in certain tumors, frequently in combination with chemotherapy

Neoadjuvant therapy refers to treatment administered prior to the definitive treatment modality
- This may shrink tumors and allow less extensive surgical intervention.
- Neoadjuvant therapy confers the same benefits of adjuvant therapy in potentially decreasing risk of both local and distant recurrence.
Factors in selecting therapy
Selecting appropriate cancer therapies is a complex task that requires a delicate balance of multiple factors. To make informed treatment decisions, healthcare providers must consider the specific characteristics of the tumor, including its type, stage, and biological features, which guide the choice of therapeutic modalities. However, equally vital is taking into account patient preference, as it acknowledges that treatment choices should align with individual values and goals, addressing quality of life and side-effect concerns. Additionally, a patient’s performance status, which assesses their overall well-being and functional abilities, plays a critical role in determining the feasibility of aggressive treatments. Finally, organ-specific considerations come into play, as the choice of therapy must consider the potential impact on vital organs and their function. By integrating these factors and employing a patient-centered approach, medical professionals can tailor cancer therapies to the unique needs of each individual, optimizing both treatment efficacy and patient quality of life.

Performance status
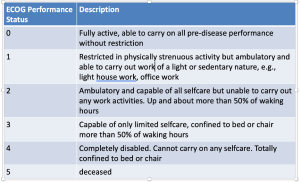
In 2012, the American Society of Clinical Oncology published their top five recommendations to improve cancer care and reduce costs, the first of which says: “Do not use cancer-directed therapy for patients with solid tumors who have the following characteristics: low performance status (3 or 4), no benefit from prior evidence-based interventions, not eligible for a clinical trial, and with no strong evidence supporting the clinical value of further anticancer treatment.” Certain highly chemo-sensitive solid tumors are rare exceptions to the general rule of not treating patients with ECOG PS 3-4
• Small cell lung cancer
• Testicular cancer
• Tumor with specific mutations predicting high likelihood of response to agents with low toxicity profiles
• Many hematologic malignancies
Tumor characteristics
• The characteristics of a tumor, including its type, stage, and biological features, along with its tissue of origin, are critical factors that significantly influence therapy selection in cancer treatment.
• The stage of the tumor also plays a pivotal role in therapy selection. In colorectal cancer, for example, early-stage tumors may be effectively treated with surgical resection alone. However, in advanced stages with lymph node involvement or distant metastases, combination therapies involving surgery, chemotherapy, radiation, and targeted therapies are often required. In metastatic disease, treatment is often LESS aggressive, since therapies are indefinite and designed to control rather than eradicate disease.
Organ specific considerations
This may seem similar to performance status, and indeed the two are often correlated. However, some cancers occur in organs that are already compromised, often by the same environmental or behavioral factors that led to the cancer itself, e.g., a patient with lung cancer may also have COPD that limits therapeutic options even if the tumor itself is theoretically curable. Another example of discordance is renal function – a patient with a kidney transplant may have an excellent performance status, but the presence of that organ – and the medications they may take to maintain it and prevent rejection – will influence treatment choice, as many systemic agents are renally cleared.

Patient characteristics
Patient characteristics – environment, home situation, resources, values, preferences – all plays key roles in shaping cancer therapy selection. On an individual level, patients and providers can engage in shared decision making around choices that best reflect the patient’s values and priorities. For example, a patient with localized prostate cancer may choose active surveillance over immediate treatment if they prioritize avoiding potential side effects like impotence or incontinence associated with surgery or radiation therapy. Patients differ in terms of their support at home, their ability to keep track of complex treatment regimens. Many patient characteristics actually represent barriers to care, which may fall outside the patient’s control. These include financial constraints, geographic distance from healthcare facilities, lack of health insurance, health literacy, and other issues. Together these can lead to disparities in cancer outcomes, which will be discussed in greater depth in the Cancer Disparities lecture later in the block. These disparities can be observed globally, in differences in diagnostic and therapeutic op=ons available to patients in low- and middle-income countries (LMICs) vs wealthy nations; they can also be observed within our own country across various groups. As we encounter patients with cancer, it is important to identify which patient characteristics pose barriers (and of these which barriers are modifiable), and which characteristics simply represent patient variation and preference.


New therapeutic trends
Personalized oncology: A medical model that separates patients into different groups (oSen based upon biologic characteristics of their tumor) – with medical decisions, practices, interventions and/or products being tailored to the individual patient based upon their predicted response or risk of disease.
• Somatic mutations in tumor to select therapy
• EGFR pathway alterations in lung and colorectal cancer
• ALK mutations (gene fusions) in lung cancer
• BRAF activating mutations in melanoma
• Germline mutations to predict therapeutic benefit
• PARP inhibitors in BRCA mutated pa=ents with breast cancer
• Protein expression on tumor to select therapy
• Estrogen receptor and Estrogen Depletion in Breast cancer
• HER2 expression in breast and gastric cancer
Media Attributions
- Screenshot 2025-06-23 at 12.49.50 PM
- Screenshot 2025-06-23 at 12.46.59 PM
- Screenshot 2025-06-23 at 12.47.53 PM
- Picture1
- Picture1
- Picture1
- Screenshot 2025-06-23 at 12.45.19 PM
- Screenshot 2025-06-23 at 12.44.50 PM
- Screenshot 2025-06-23 at 12.42.40 PM
- Picture1
- Screenshot 2025-06-23 at 12.41.28 PM
- Screenshot 2025-06-23 at 12.41.05 PM
- Screenshot 2025-06-23 at 12.40.39 PM
- Screenshot 2025-06-23 at 12.39.44 PM
- Enter your footnote content here. ↵