9 Venous Thromboembolic Disease
Student Learning Objectives
- Recognize the pathophysiology of venous thromboembolic disease.
- Identify congenital and acquired risk factors associated with increased thrombotic risk.
- Translate the clinical features and laboratory evaluation of antiphospholipid antibody syndrome.
- Apply clinical or laboratory factors that influence anticoagulant selection in the management of venous thromboembolism.
- Recognize heparin-induced thrombocytopenia and discuss appropriate management.
Introduction
Throughout your career as a physician, you will encounter patients with blood clots. These can be provoked blood clots, such as clots caused by vascular trauma or venous stasis, or can be unprovoked blood clots. There are inherited disorders that predispose patients to clotting. There are also acquired problems, such as certain acquired antibodies that can be associated with blood clots. We have covered treatments for blood clots (and their reversal agents) in a separate course pack/lecture. Now we’ll discuss clinical scenarios and drugs used in those scenarios for treatment.
Arterial vs Venous Blood Clots
Blood clots or thromboses in the vascular system interrupt blood flow. In arterial systems, this leads to ischemia (think of heart attacks or strokes as examples). In the venous system, these clots disrupt venous return of blood flow to the heart. Clots in the venous system can break off and travel back through the heart to the lungs and thus cause a pulmonary embolus.
Arterial blood clots are occurring generally in arteries, which have a relatively high flow of blood. These clots are usually associated with plaque buildup (atherosclerosis) in the vessel and platelet plugging. Hence, they are often called “white thrombi”. In these types of clots, the primary clotting system is usually the more active system and fibrin (secondary hemostasis) formation is less problematic. These clots are generally treated with antiplatelet agents (such as aspirin that inhibits platelet function), and you will hear more about these types of clots in your cardiovascular block learning.
Venous blood clots occur in veins, which is generally a lower flow state. These clots often are due to low flow/stasis in veins with activation of the secondary clotting cascade. These clots can be referred to as “red thrombi”, and fibrin formation is usually more active than platelet activation in causing these types of clots. Hence, these types of clots are generally treated by inhibiting the clotting cascade via use of anticoagulants.
 The remainder of our focus for this topic will be on venous clots. Arterial clots are covered in the block on cardiovascular disease.
The remainder of our focus for this topic will be on venous clots. Arterial clots are covered in the block on cardiovascular disease.
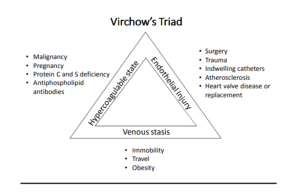
Virchow’s Triad
Blood clots in the venous system generally are due to 3 problems, referred to as “Virchow’s Triad”. These include Stasis of blood (slow flow or no flow), Endothelial injury to the vessel wall, and hypercoagulability.
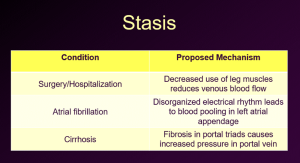
Venous stasis
This occurs in the venous system when muscles around blood vessels aren’t contracting to help move the blood along the and in the portal venous system when scarring the liver (such as cirrhosis) leads to back up of blood in the portal vein. Examples include the following:
Endothelial Injury
Endothelial Injury to the venous endothelial wall can lead to clotting. This can be caused by direct trauma to the inner wall of the vein, such as that caused by a central venous catheter or other blunt trauma. Alternatively, drugs that are toxic to endothelial cells (the classic example is that of chemotherapy drugs) can cause toxic injury.
Hypercoagulability
due to inherited or acquired disorders that contribute to increased activation of the clotting cascade are the 3rd arm of Virchow’s Triad. These include the following:
(Note: “Pro C” refers to protein C and “Pro S” refers to Protein S. As you recall from your clotting cascade lecture/course pack, protein C, protein S, and antithrombin are involved in suppressing the clotting cascade).
The above are all risk factors for developing venous blood clots. Of note, the venous blood clots in superficial veins are called thrombophlebitis and are usually not of clinical importance, and often don’t need to be treated. Blood clots in deep veins are at risk of breaking off and embolizing to the lungs. Hence, when we talk about blood clots that require treatment, we are referring to deep vein thromboses (DVTs) or pulmonary emboli (PEs).
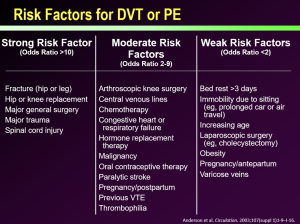
Risk factors for DVT/PE
Risk factors for DVT and/or PE can be characterized as strong risk factors or weaker risk factors for developing a DVT/PE. From many years of experience in medicine, we have been able to stratify these risk factors as follows:
When to suspect DVT or PE
DVT and PE are both easy to miss. Patients can have no symptoms. It is important to keep the diagnosis of DVT and PE in the back of your mind when assessing patients. However, when patients do present with symptoms, these are classic:
DVT: Unilateral pain and/or swelling of an extremity.
PE: Pleuritic chest pain and/or dyspnea that is often of sudden onset. Sudden tachycardia or hypoxemia also often are seen in this setting.
If you have a patient with these symptoms, and especially a patient with other underlying risk factors such as recent surgery, estrogen exposure, trauma, cancer, chemotherapy, etc), make sure you rule out DVT/PE.
How to treat a patient with DVT/PE
Treatment of a patient with DVT and/or PE has both short-term and long-term goals. The short-term goals are to control symptoms and prevent extension (propagation) of the clot that has formed, prevent embolization of a clot and death, and prevent new clots from forming.
Long-term goals are to prevent recurrent thrombosis, reduce the risk of post-thrombotic symptoms, and prevent death from recurrent pulmonary emboli.
The acute phase of treatment of a DVT/PE involves quickly anticoagulating the patient to achieve short term goals. The chronic phase of treatment of a DVT/PE involves ongoing anticoagulation to further achieve short term goals and achieve long term goals. Since the 1990s, treatment of DVT/PE in the acute phase and chronic phase has undergone quite a change with the advent of new drugs.

Prior to the 1990s, we had essentially 2 drugs. IV heparin was used to immediately anticoagulated patients with clots. This had to be done in hospital setting, since heparin requires close monitoring and is only effective in the acute setting when given IV to rapidly anticoagulated patients. These patients were in the hospital on heparin and then converted to oral warfarin. Heparin could not be stopped until after warfarin was at a therapeutic INR (INR between 2-3) for 2 days. At that point, patients could be discharged on warfarin with close follow up with their PCP or an anticoagulation clinic to monitor the warfarin levels.

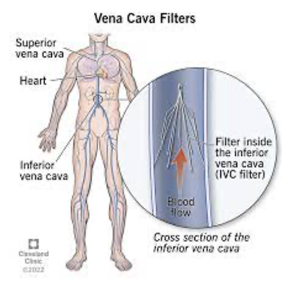
With the advent of newer drugs, we now have other options to IV heparin. Hence, patients can be treated as outpatients, if their symptoms aren’t severe and they are compliant with medications. For large clots, we also have options to lyse such clots, which is generally done under the care of interventional radiologists. In patients with a DVT or non-fatal PE who have absolute contraindications to anticoagulation (think of a patient with active GI bleeding as well as a DVT/PE), Interventional Radiology can place a filter in the Inferior Vena Cava to catch large clots embolizing from lower extremities, to at least mitigate the chance of a large PE causing death.
In summary, Anticoagulation is the mainstay of treatment for most patients with DVT/PE. Thrombolysis is used in more extreme settings in patients with significant compromise due to clot, such as PE with hypotension and shock due to decreased blood return to the heart and in the setting of Proximal DVT of a limb with severe compromise of tissue perfusion due to back pressure from the clot. As noted above, IVC filter placement is used in place of anticoagulation in settings where a patient has an acute blood clot and an absolute contraindication to anticoagulation therapy.
Anticoagulant drugs
We have previously discussed anticoagulant drugs in prior course pack material and lectures. We review the options for treatment in the following graphic.
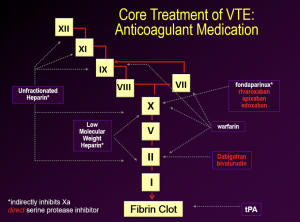
Treatment choices are generally made by physicians while taking into account their patient’s preferences, drug administration, insurance coverage, and half-life of the drug if the patient is also going to be undergoing invasive procedures in the future.
Identifying and mitigating risk factors for blood clots
Once a blood clot has been diagnosed and urgent treatment initiated, it is important to try to identify the underlying cause for the clot. Obviously, if the patient has known risk factors such as oral contraceptive use, immobility, etc, then the clot is thought to be provoked by these factors. Generally, anticoagulation is continued for 3 to 6 months, and any factors thought to be provoking the clot are mitigated prior to stopping anticoagulation.
Inherited and Acquired Thrombophilias
In patients with unprovoked clots (clots that seem to occur despite no risk factors for such clots), it is important to screen the patient for acquired or inherited thrombophilias.
Inherited thrombophilias (inherited disorders that increase risk of clotting) include the following:
- Antithrombin deficiency: Deficiency of antithrombin. Recall that antithrombin acts with natural heparins in the body and is important for slowing down/controlling the clotting cascade and binds to Xa and thrombin. Deficiency of antithrombin increases risk for clotting.
- Protein C deficiency: Recall that protein C is involved in slowing down/controlling the clotting cascade by binding with protein S and inhibiting factors VIIIa and Va. Deficiency of protein C increases risk for clotting.
- Protein S deficiency: Recall that protein S is involved in slowing down/controlling the clotting cascade by binding with protein C and inhibiting factors VIIIa and Va. Deficiency of protein S increases risk for clotting.
- Factor V leiden gene mutation (heterozygote or homozygote): Factor V leiden is a mutation that increases the activity of factor V. Increased activity of factor V increases the risk for clotting.
- Prothrombin 20210A gene mutation (heterozygote or homozygote): This mutation increases the biosynthesis of prothrombin (factor II). As a result, individuals with this mutation have approximately 30% higher plasma levels of factor II, and this has been associated with increased risk of venous thrombosis.
Acquired Thrombophilias are disorders of clotting due to problems that individuals have acquired over their lifetime and include the following
- Cancer: Cancers are associated with risks for blood clots. Interestingly, clots occurring despite therapeutic treatment with warfarin are more common in the setting of cancer.
- Development of antiphospholipid antibodies: These antibodies can develop on their own or in the setting of a connective tissue disorder, such as lupus. There are a variety of antiphospholipid antibodies. The ones most commonly associated with risks for blood clots are anti-cardiolipin antibodies and ant-beta-2-glycoprotein-1 antibodies. These antibodies are associated with both venous and arterial thrombosis.
Question: Why do we want to know if clots were provoked or unprovoked and whether there are any associated thrombophilias?
Answer: This information will impact decisions about the type of anticoagulation used, duration of anticoagulation, and whether it is “safe” to stop anticoagulation after a certain period of time on anticoagulation.
Patients that have blood clots that are caused by surgery or are provoked by factors that can be mitigated (such as stopping oral contraceptive use if the clot was thought to be provoked by oral contraceptives), are much less likely to have recurrent blood clots after anticoagulation is stopped.
Patients who have unprovoked blood clots are more likely to have recurrent blood clots after anticoagulation is stopped.
When to stop anticoagulation/treatment after a patient has had a blood clot and been treated for it
It is important that shared decision making occur with patients in deciding if and when to stop blood thinners. Information about underlying causes for the patient’s clot(s) plays into this shared decision making. Risk for recurrent blood clots after stopping anticoagulation vs risk from bleeding due to ongoing use of anticoagulants needs to be frankly discussed in light of every patient’s individual clinical situation.
Example of shared decision making: A patient is diagnosed with a leg DVT a few months after starting oral contraceptives (OCPs) for birth control. The birth control pills are stopped and she is anticoagulated on rivaroxiban for 3 months. At the end of 3 months of anticoagulation she is doing well, her DVT has resolved, and she plans to not resume use of OCPs for birth control, but instead switch to a copper IUD. In this case, the blood clot she had was likely provoked by OCP use, and the provoking drug (OCP) has been stopped. The clot was not life threatening and is not likely to recur. You and the patient both feel that the benefits of further anticoagulation are outweighed by risks of bleeding from ongoing anticoagulant use, and hence anticoagulation is stopped.
Another example of shared decision making: A long haul truck driver who drives 12 hours a day for his job is diagnosed with a DVT and a life-threatening PE. He is anticoagulated, and he recovers. He is not able to change jobs and needs to continue his job as a long-haul truck driver. The risk factor cannot be mitigated. He has no problems with the anticoagulant use. You and the patient decide that he will continue anticoagulation indefinitely as long as he works as a long-haul truck driver and continues to have no problems with his anticoagulant medication. The patient will come back to see you if/when he retires from long-haul truck driving, and you will re-assess at that time if anticoagulation can be stopped.
Risks of Anticoagulant use
When treating patients with anticoagulants due to DVT/PE, it is important to be aware of risks from these drugs.
- Bleeding: All anticoagulants carry a risk for bleeding. Bleeding risks are higher in patients with advanced age, renal impairment, uncontrolled hypertension, use of other antithrombotic medications such as antiplatelet agents, NSAID use, and history of stroke. It is important that patients on these drugs be counseled to seek medical attention for active bleeding that doesn’t stop, signs of GI bleeding (dark stools, blood in stools, etc.), and head trauma that could result in head bleeding. It is also important to consider what reversal agents patients will have access to if they have life-threatening bleeding on their anticoagulant.
- Warfarin skin necrosis: A rare but serious risk with warfarin use is warfarin skin necrosis. Recall that Warfarin inhibits not only factors X, IX, VII, and II, but also inhibits protein C and protein S, which are important in control of the clotting cascade. Recall as well that when warfarin is first started, it can be thrombogenic, due to inhibition of protein C and S, until the warfarin is fully therapeutic with an INR of 2 to 3 for 48 hours. Individuals with protein C deficiency are particularly at risk for warfarin-induced skin necrosis. Patients with this problem present with painful ecchymoses in fat-containing areas of the body such as the breasts or buttocks (but other areas can be involved as well). Microscopically, these lesions contain fibrin thrombi within venules, accompanied by hemorrhagic necrosis.
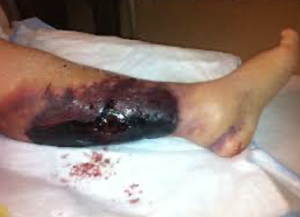
- Heparin-induced thrombocytopenia: Heparin-induced thrombocytopenia (HIT) is a life-threatening disorder. Patients on heparin present with a sudden drop in platelets. This is associated with a high risk of thrombosis: 30-50% of patients with this disorder who are only treated with withdrawing heparin develop thromboembolic events in the first 30 days after this diagnosis is made. It is imperative that heparin be stopped. Unless the patient has active bleeding, anticoagulation using a non-heparin agent should be started asap, regardless of the platelet count.
The cause of HIT is the development by the patient of an autoantibody that is directed against a complex of heparin and platelet factor 4 released from platelet alpha granules. The antigen-antibody complex bonds to Fc receptors on platelets and triggers their activation. This results in thrombocytopenia but also leads to serious venous and arterial thromboses in a significant percentage of patients. Along with stopping heparin, anticoagulation with non-heparin agents such as fondaparinux or IV direct thrombin inhibitor (argatroban or bivalirudin, and then convert to warfarin after the acute setting has resolved).
HIT Mechanism and Treatment
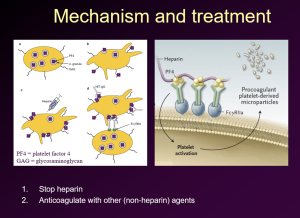
We have talked about a variety of inherited and acquired thrombophilias, as well as management of blood clots and complications of anticoagulant therapies.
We are now going to transition another topic to focus on one of the acquired thrombophilias: Antiphospholipid Antibody Syndrome. The presence of antiphospholipid antibodies by themselves in individuals is common. We certainly don’t anticoagulated people simply because they are found to have anti-phospholipid antibodies….
Antiphospholipid Antibody Syndrome
Antiphospholipid Antibody Syndrome (APS): This disorder is characterized by thrombosis in association with persistent presence of antiphospholipid antibodies anti-cardiolipin I or anti-beta-2-glycoprotein I as measured by repeated lab tests 12 weeks apart.
Note that antiphospholipid antibodies cause a prolonged PTT (partial thromboplastin time) and can also cause a prolonged PT (prothrombin time) because they interfere with the assays. Unlike the setting of a factor deficiency causing a prolonged PTT or PT, the prolonged PTT or PT in the setting of antiphospholipid antibodies is not “corrected” by the addition of normal plasma with a “mixing” study. Despite the prolonged PTT and/or PT, antiphospholipid antibodies carry a risk for thromboses. Patients who develop thromboses (arterial or venous) and have these persistent antibodies are diagnosed with Antiphospholipid Antibody syndrome. Other clinical manifestations of APS include immune thrombocytopenia and recurrent fetal loss. Additionally, once may see autoimmune hemolysis, unexplained renal disease with thrombotic microangiopathy on kidney biopsy, unexplained cardiac valve vegetation or thickening, and skin findings called Livedo racemose or Livedo reticularis.
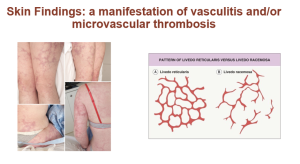
The disorder is diagnosed in the setting of clotting/typical clinical findings by testing for specific antiphospholipid antibodies: certain Anticardiolipin antibodies and anti-beta-2-glycoprotein I antibodies. Antiphospholipid antibodies that prolong the PTT are often termed “lupus anticoagulants”, although these antibodies can be present in patients with out lupus (and not all patients with lupus have these antibodies). There are additional “lupus anticoagulant” assays that are ordered when evaluating for APS.
Patients diagnosed with APS should be treated with anticoagulation. In patients with suspected APS, warfarin is typically preferred over DOAC drugs, particularly in patients with “high risk profile aPL lab tests”. This is because there is evidence that DOACs are less effective than warfarin for recurrent thrombosis prevention in patients with APS. Hence, patients are often anticoagulated initially with heparin or LMW heparin and then transitioned to warfarin.
Of note, High-risk aPL profile lab findings include the presence of a lupus anticoagulant, double positive antiphospholipid antibodies (any combination of Lupus anticoagulant, anticardiolipin antibodies or anti-beta-2-glycoprotein 1 antibodies), or triple positive APL (all three aPL tests positive).
For patients with APS in the setting of an arterial clot, generally warfarin plus aspirin is utilized in this setting.
More severe forms of APS, aka “catastrophic APS” are also treated with immunosuppression to mitigate antiphospholipid ab production and plasma exchange to the remove the antibodies in the acute setting.
Authors and Contributors:
Corliss Newman, MD wrote this to correspond with the pharm lecture prepared by David Garcia, MD. Several of Dr. Garcia’s slides were incorporated into this course pack material.