2 Erythropoiesis and the Red Blood Cell (RBC)
Learning Objectives
- Explain the process by which a hematopoietic stem cell differentiates into a mature red blood cell.
- Identify the factors that impact the affinity of hemoglobin for oxygen.
- Describe the hypoxic regulation of erythropoiesis via erythropoietin and erythropoietin receptor signaling.
- Recognize the organization and function of the major proteins of the red cell membrane and cytoskeleton.
- Give examples of defects in normal red blood structure and metabolic pathways that lead to disease.
USMLE Content Descriptors: Cell structure and function. Hemolysis. Disorders of hemoglobin, heme or membrane. Cytopenias.
Erythropoiesis
Here we aim to give an overview of red cell production including where it occurs, RBC lifespan, key drivers that regulate this process. We also aim to summarize RBC structure and key and metabolic pathways critical for RBC function, which is mainly O2 delivery (and removal of CO2). We also briefly discuss mechanisms of RBC removal.
First, we focus on what drives hematopoietic stem cells (which we discussed in our prior lecture) to specifically differentiate into red blood cells. Key to understanding this process is the concept that the purpose of red blood cells is to carry oxygen from the lungs to organs and tissues and also transport carbon dioxide in the reverse direction. To do this effectively, Red blood cell (RBC) production is tied to oxygen needs/hypoxia. Also, the design of the RBC/it’s shape is critical for it to be able to get to areas that need oxygen via capillaries. We’ll look at how affinity of the hemoglobin in the RBC for Oyxgen is affected by hypoxia/pH, etc. We’ll also look at how defects in red blood cell structure and metabolic pathways can lead to certain medical disorders.
Erythropoiesis is the process by which hematopoietic stem cells become committed to production of erythroid progenitor cells and these then mature into mature red blood cells. There are several cells along this pathway of differentiation/maturation of red blood cells.
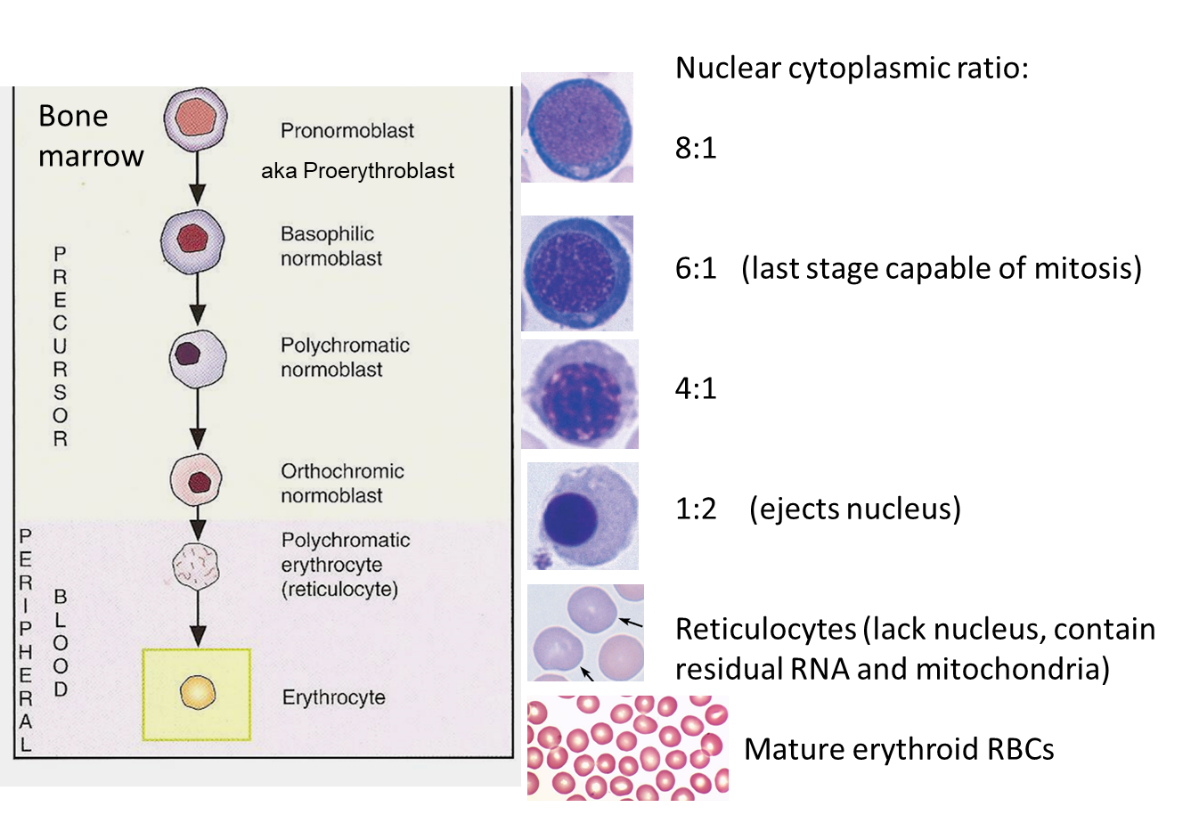
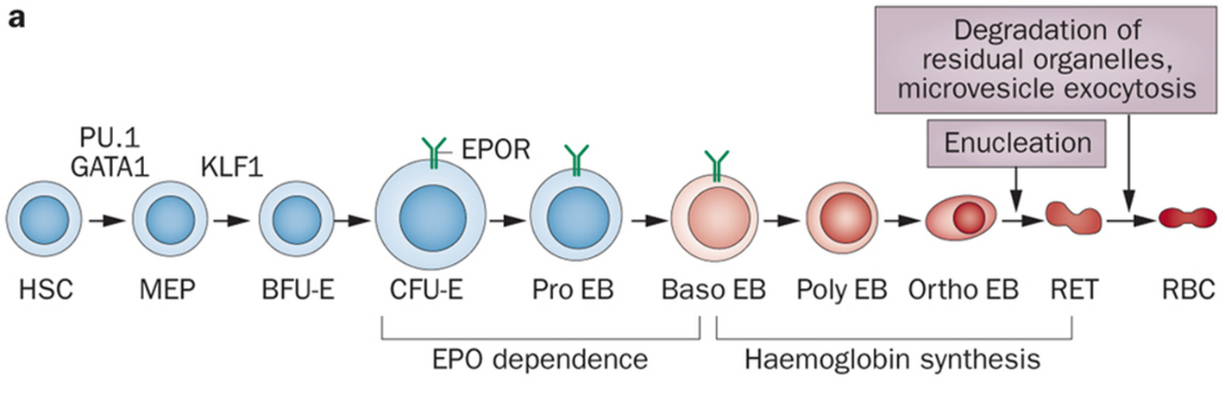
Abbreviations for reference:
HSC: Hematopoietic Stem Cell
MEP: Multipotent Erythroid Progenitor cell, aka Bipotent megakaryocytic -erythroid progenitors)
BFU-E: Burst-forming unit-erythroid cell
CFU-E: Colony-(forming unit-erythroid cell
Pro EB: Pro-erythroblast cell
Baso EB: Basophilic erythroblast cell
Poly EB: Polychromatic erythroblast cell
Ortho EB: Orthochromic erythroblast cell
RET: Reticulocyte–an enucleate red blood cell that still has some mRNA present in the cytoplasm. Note 20% of hemoglobin is produced in the reticulocyte. Reticulocytes assume mature RBC shape (biconcave disc) within 1 to 2 days after repeated passage through the spleen (grooming).
RBC: Red Blood Cell (the final product in the pathway).
When looking at a bone marrow specimen and identifying cells involved in Erythropoiesis….
On a H&E stain slide of bone marrow, the hematopoietic stem cells thru CFU-E cells are not identifiable—they all look like undifferentiated blasts.
Pro-erythroblasts can sometimes be noted on a H&E slide of bone marrow, as they typically have a more brilliant blue cytoplasm and more abundant cytoplasm than other blasts.
As the various types of erythroblasts mature and start to make hemoglobin, their cytoplasm changes color (on and H&E-stained slide) from blue to pink (Think about why this occurs—answer, hemoglobin is being produced as the cells mature). Also, the nucleus of these cells becomes progressively smaller and then is extruded from the cell between the ortho EB stage and the reticulocyte stage.
Note, a hormone produced by the kidneys, erythropoietin, acts to help drive red blood cell production, and this is active on red cell precursors in the bone marrow in the CFU-E thru Baso EB stage of Erythropoiesis.
In the bone marrow, Erythropoiesis is promoted by cytokines and occurs around specialized macrophages (remember macrophages are derived from monocytes, recall prior lecture, and in this case these particular macrophages in the bone marrow are secreting cytokines that stimulate Red Cell Production as part of their work in the bone marrow). These specialized macrophages not only supply cytokines needed for RBC development/maturation, but also help anchor red cell precursor cells, AKA erythoblasts (some people use the term “normoblast” in place of the term “erythroblast) to the bone marrow stroma (this is probably why you don’t normally see these precursor cells circulating in blood). Also, macrophages are part of the reticuloendothelial system and store iron, and bone marrow macrophages are where the iron is stored in bone marrow to then be supplied to RBC precursors for heme synthesis.
Next, we move on to talk specifically about the production of hemoglobin by cells, as they mature into RBCs, which is critical for RBC purpose/function:
Hemoglobin, the protein that carries Oxygen, is produced from the Baso EB stage thru the RET (reticulocyte) stage of RBC production/maturation. Hemoglobin has 2 parts. The heme part is the part that has iron incorporated and carries O2. The globin part of hemoglobin is the protein part. There are 4 globin proteins (most commonly 2 alpha and 2 beta chains, more on this later) that bind in a conformation to hold 4 heme particles to make a complete hemoglobin particle. RBCs are full of hemoglobin to carry oxygen. Erythropoiesis, the production of red blood cells, requires adequate heme and globin production.
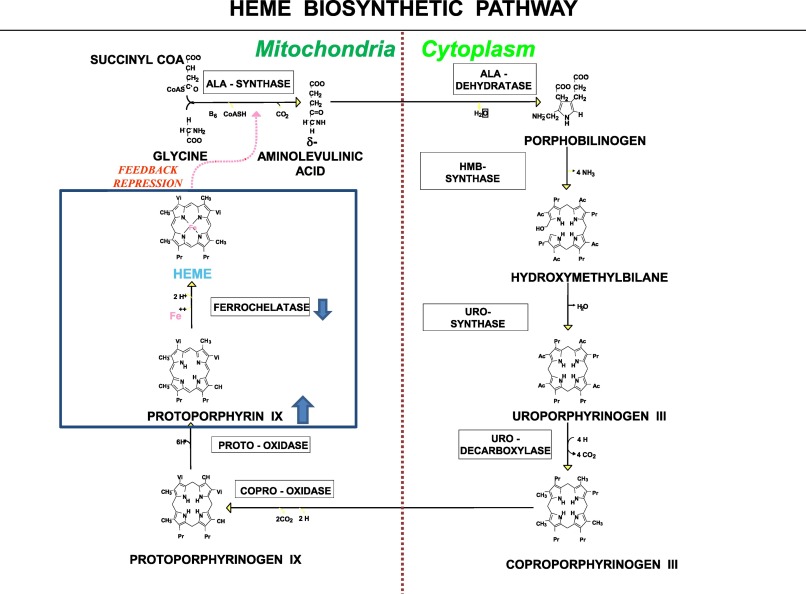
Heme Biosynthesis
Heme biosynthesis—the process of producing the heme moiety—is a multistep process. Heme biosynthesis involves 8 enzymes, four of which are cytoplasmic (occur in the cytoplasm) and four of which are mitochondrial. The initial step takes place in the mitochondria, where succinyl-CoA and glycine, in the presence of the enzymes 5-aminolevulinic acid synthase-2 (ALA-S2) yields delta-aminolevulinic acid (ALA). This first step is the rate-limiting step for heme biosynthesis. The last step, where iron is incorporated into the heme moiety, involves the enzymes ferrochelatase, and is the 2nd rate limiting step in this process. Interestingly, both the first and last steps occur in the mitochondria.
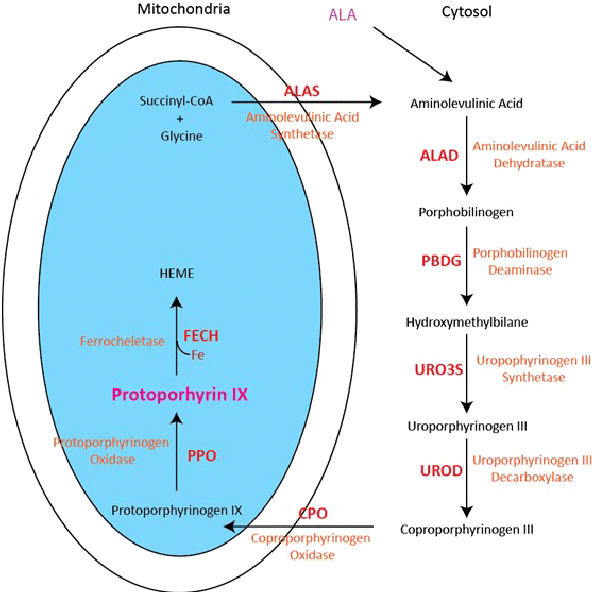
Note: You do not need to know all the details of this heme biosynthetic pathway. You should know the enzymes and products involved in the first and last steps of production, which are the 1st and 2nd rate limiting steps! The entire pathway above is shown to give you some context, as the different porphyrias (see below) are determined by where in the above pathway heme synthesis is disrupted.
Each step in the process is controlled by a different enzyme. As you can imagine, defects in heme synthesis, often the result of mutations affecting enzyme function along the multi-step pathway, can result in certain diseases.
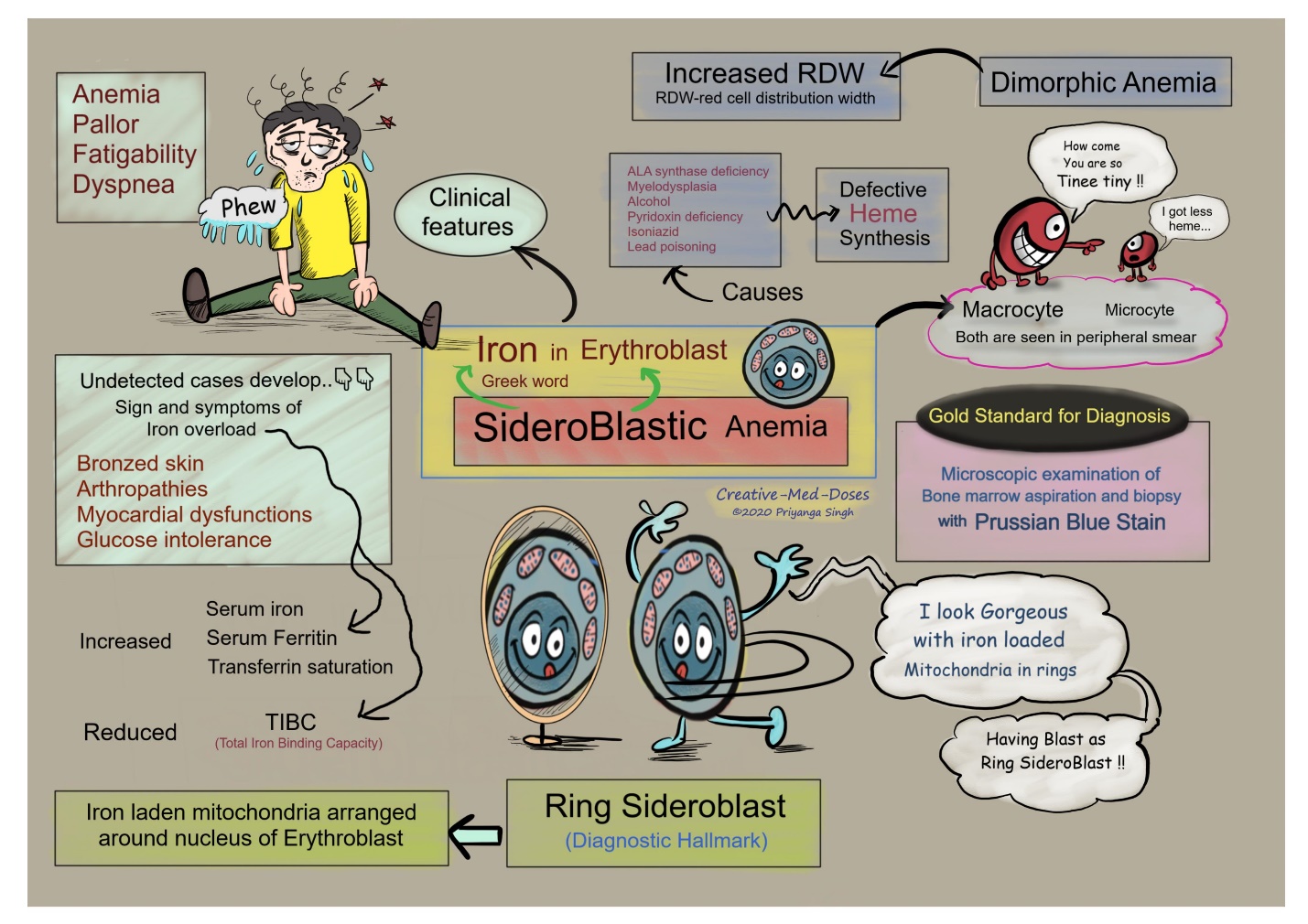
- X-linked sideroblastic anemia: This is a congenital anemia, X-linked recessive, that is due to mutations in ALA-S2, an erythroid-specific isoform of the first enzymes in the heme synthetic pathway. Vitamin B6 is a cofactor for ALA-S2, and hence B6 supplements can be helpful. The Anemia is labelled Sideroblastic because impaired heme synthesis leads to presence of iron granules around the nucleus of red blood cell precursors, presumably because the iron can’t be incorporated into the heme moiety due to the mutation. Cells with iron granules around them can be seen with a certain stain on bone marrow, and these are called “ringed sideroblasts”. Ringed sideroblasts are seen in other certain types of anemia, but are certainly present in this type, hence the name.
- Porphyrias: Porphyrias are a group of disorders with various clinical symptoms that are due to defects in specific heme biosynthetic enzymes. Each different porphyria results from a defect in one of the specific heme biosynthesis enzymes. This causes products (intermediates) in the heme biosynthesis pathway to accumulate in the cells, and this can lead to certain disorders.
Prophyrias are generally classified by whether they cause neurovisceral symptoms or photocutaneous symptoms. Some types (2) have both neurovisceral and cutaneous symptoms.
You are not responsible for knowing all the different prophyrias, but if you are interested, here they are:
-
- Neurovisceral symptom porphyrias: Aminolevulinic Acid Dehydratase Deficiency Porphyria, Acute Intermittent Porphyria.
- Mixed neurovisceral symptom and photocutaneous symptom prophyrias: Hereditary Coproporphyria, Variegate Porphyria.
- Photocutaneous symptom prophyrias: X-linked Dominant Protoporphyria, Congenital Erythropoietic Porphyria, Porphyria Cutanea tarda, Erythropoietic Protoporphyria, Hepatoerythropoietic Porphyria.
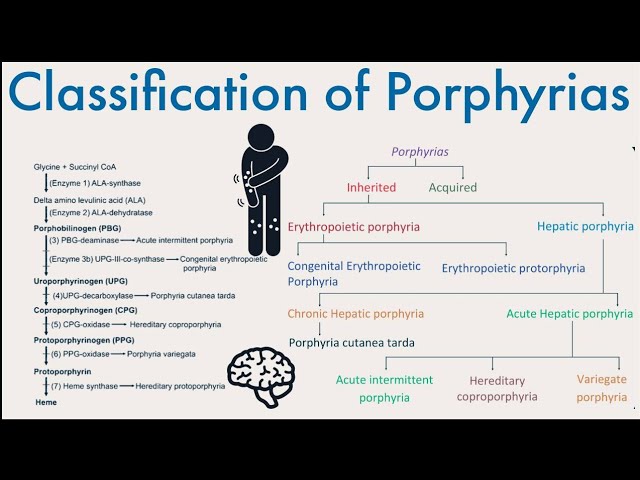
You are not responsible for knowing all of these porphyrias
You should be aware of: Erythropoietic Protoporphyria (EPP)
Erythropoietic Protoporphyria (EPP): This is due to deficient activity of ferrochelatase, the last step in heme synthesis where iron is incorporated into Protoporphyrin IX (PPIX). This causes Protoporphyrin IX to build up in the body. Zinc can be incorporated into the protorophyrin ring in place of iron (such as in iron deficiency), however the deficiency in ferrochelatase also effects incorporation of zinc, so there is a build up of metal-free protoporphyrin. Excess PPIX in blood/skin exposed to visible light causes activation of O2 species and photosensitivity. This is an autosomal recessive disorder, is the most common porphyria in kids, and causes acute non-blistering photosensitivity. (Note, there is another type of porphyria that causes blistering, hence important to note that EPP is non-blistering.) PPIX is not water soluble so porphyrins are not detected in urine.
Acute Intermittent Porphyria: This type of porphyria, which causes neurovisceral symptoms such as abdominal pain and confusion, is due to low levels of the enzyme porphobilinogen deaminase, causing build-up of the intermediate prior to that step of heme synthesis. AIP is famous as the cause of the madness of King George (of England) and was also a featured disorder in Season 1 of House, episode 2 Honeymoon.
Porphyria Cutanea Tarda: Porphyria cutanea tarda-PCT, is one of the hepatic porphyrias. PCT is associated with deficiency in UROD enzyme, the 5th catalytic step of heme biosynthesis that occurs in the cytosol. There may be genetic mutation leading to deficiency and/or acquired factors such as ETOH use and hepatitis C. PCT is associated with iron-dependent UROD insufficiency and can respond to phlebotomy (removal of whole blood) to deplete iron stores (reducing ferritin levels).
We now move on to discuss hypoxic regulation of erythropoiesis via erythropoietin (EPO) and erythropoietin receptor signaling:
Hypoxic regulation of erythropoiesis via erythropoietin (EPO) and erythropoietin receptor signaling:
Erythropoietin is produced by the kidneys when cells in the kidney sense hypoxia. EPO is then produced and travels in the blood stream. Erythroid progenitor cells in the bone marrow are stimulated by EPO to increase production of mature erythrocytes.
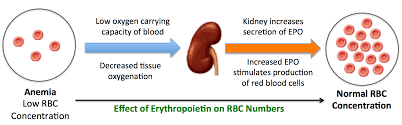
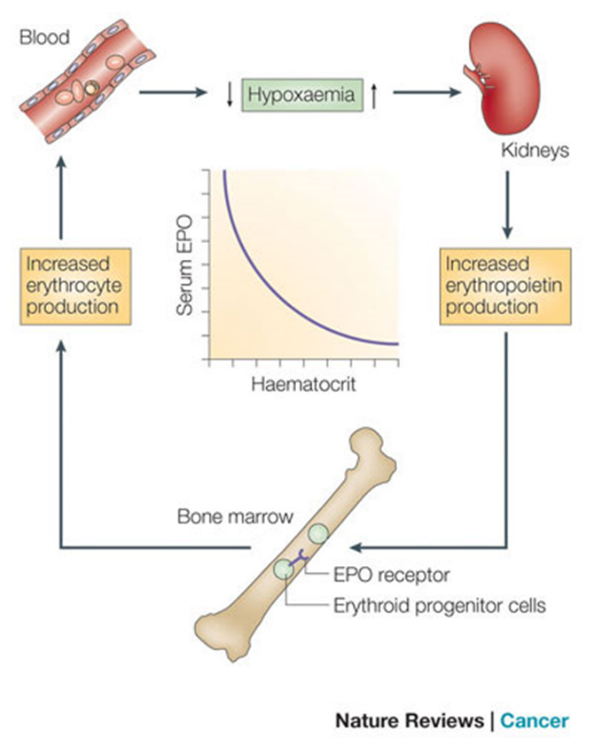
Key Concepts of EPO Production
Low oxygen environments in the body lead to EPO gene transcription. Essentially, in normoxic conditions, EPO gene transcription is inhibited. However, in settings of hypoxia, cellular events in the kidney leading to EPO production are not shut down, and EPO gene transcription is thus upregulated.
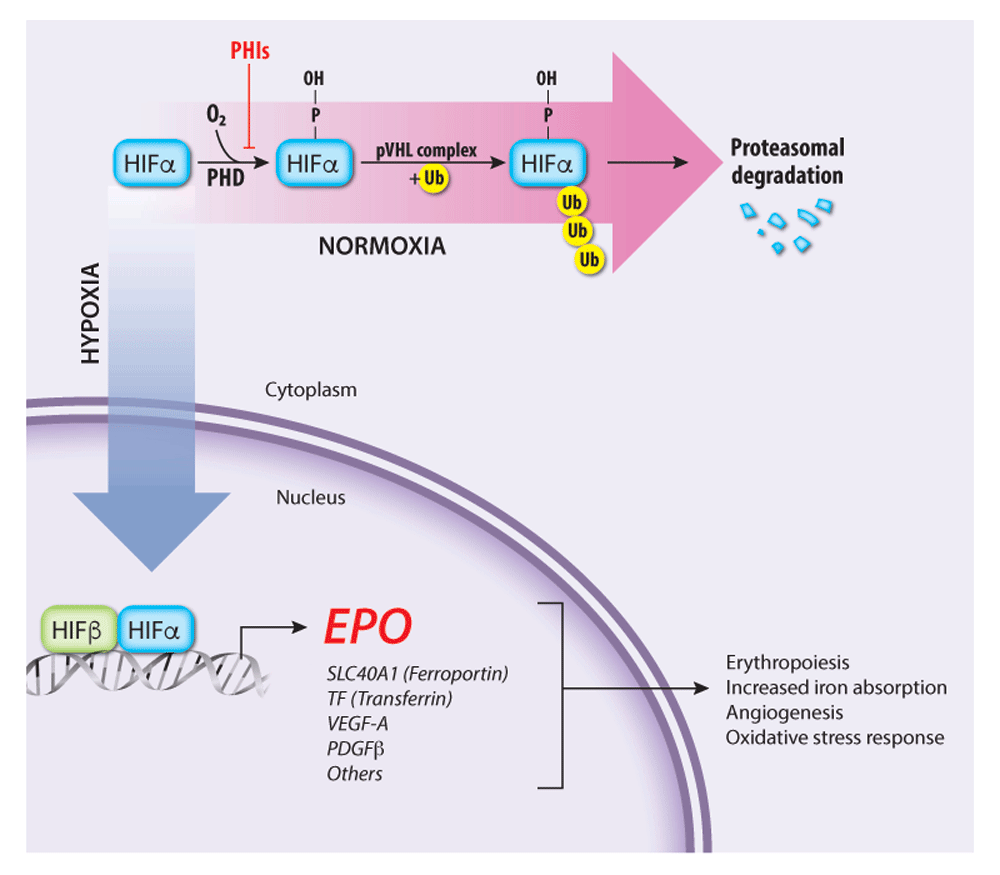
Renal peritubular cells sense hypoxia and this induces EPO production. These kidney cells sense lack of oxygen—either due to hypoxia because of low oxygen inspired or due to a lack of cells available to carry oxygen (eg anemia)–and this results increased EPO production by the kidney.
90% of EPO is produced by the kidneys.
Chronic hypoxia (eg living at high altitude) results in EPO production causing a compensatory increase in red blood cells (polycythemia).
Chronic kidney disease can cause anemia due to lack of EPO production.
EPO acts on erythroid progenitors and early precursors (CFU-E thru Basophilic-EBs) to increase RBC production. In these cells RBC precursor cells, EPO binds to EPO-receptors, which activates JAK2 signaling pathways to promote erythroid survival, proliferation, and differentiation.
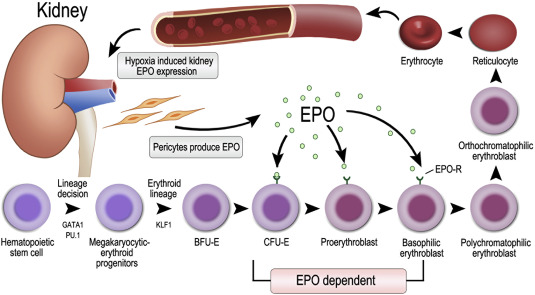
Characteristics and Function of the Mature RBC
Optional text book reading: Part 1 Anemia and Red Cell Disorders
Chapter 10: Inherited Hemolytic Disorders of the Red Cell Membrane and Red Cell Metabolism (section on disorders of the red cell membrane)
We now move on to discuss the organization/structure and function of the major proteins of the red cell membrane and cytoskeleton. Of note, although not required reading for this session, Chapter 10 of your textbook, Pathophysiology of Blood Disorders has a brief summary of the normal proteins/structure of RBCs and then talks about disorders or red cell membrane and RBC metabolism. I highly recommend you read this 9-page chapter.
RBC Characteristics: The Mature red blood cell is an anucleate biconcave disc. The biconcave shape, which is due to the specialized membrane/membrane proteins of the cell, allows a high surface to volume ratio of the cell to facilitate gas exchange. In addition, the biconcave formation allows for RBC deformability as the RBC flows through tiny capillaries. RBC deformability is further enhanced by the elasticity of the RBC membrane, and is also affected by cytoplasmic viscosity (hemoglobin concentration within the RBC). 90% of the protein concentration in this RBC is hemogobin, which functions to carry O2, as discussed. A typically RBC will live about 120 days in circulation, before being removed by the spleen as it becomes senescent.
Key membrane proteins in the RBC membrane include spectrin, F-actin, Band 3, Ankyrin, and Proteins 4.1 and 4.2. (See picture below.) Note that defects in the “vertical” connections between the membrane skeleton and band 3 result in hereditary spherocytosis (cells are sphere-shaped, as opposed to biconcave discs). Defects in the “horizontal” interactions that hold the membrane skeleton together cause hereditary elliptocytosis or a more severe variant of this called hereditary pyropoikilocytosis.
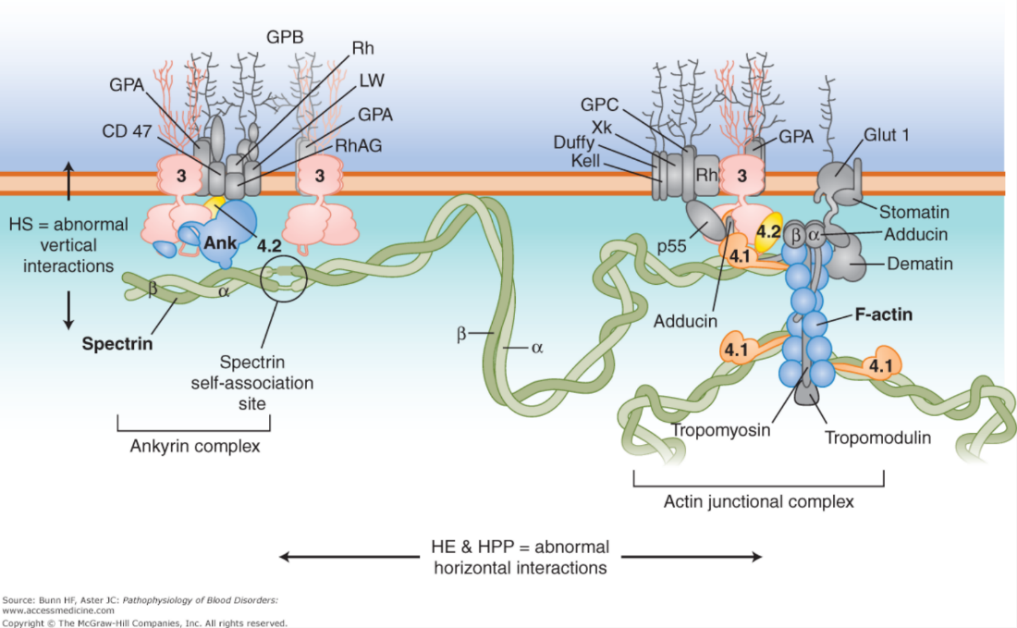
Defects in RBC membrane and deformability lead to shortened RBC survival, increased RBC turnover, and susceptibility to red blood cell lysis. Some patients require removal of the spleen which can improve red blood cell survival.
Factors that impact the affinity of hemoglobin for oxygen:
Hemoglobin can carry up to 4 O2 molecules at a time (one on each heme moiety, and there are 4 heme moieties in each hemoglobin molecule). The 4 hemoglobin molecules bind such that there is a pocket for the heme moiety and the O2 fits in this area. There are 2 factors that affect O2 binding—making it more or less likely to be/stay bound. These factors are involved in facilitating the binding of O2 to heme in the lung (and release of CO2 to the lung) and then releasing O2 in hypoxic tissues (and binding CO2 to heme in hypoxic tissues). This is critical for adequate oxygen exchange. These 2 factors are the following:
- Environment within the RBC: pH, temperature, and concentration of 2,3 BPG.
- Cooperativity through steric conformational change of the hemoglobin.
The oxygen-hemoglobin dissociation curve graphically shows the relationship between PO2 (pressure of O2) in the blood and the percent saturation of hemoglobin with oxygen (oxyhemoglobin). Basically, the curve plots the proportion of hemoglobin in its saturated (oxygen-laden) form on the vertical axis against the prevailing oxygen tension (pressure) on the horizontal axis. The sigmoid shape of this curve is due to cooperativity through steric conformational change of the heme/hemoglobin moiety. The oxygen-hemoglobin dissociation curve will be shifted to the left or right based upon the environment. This topic is discussed again in your pulmonary/respiratory system block later this academic year, but we’ll visit this issue in this session as well. Chapter 3 of your text, Pathophysiology of Blood Disorders, has a brief 2-page discussion on this issue and how anemia affects O2 carrying ability.
Note, when the curve is shifted to the right, the red cell hemoglobin releases more O2 (doesn’t hang on to O2 quite as tightly, has lower affinity for O2). When the curve is shifted to the left, the red cell hemoglobin hangs on to O2 a bit more tightly (has more affinity for O2).
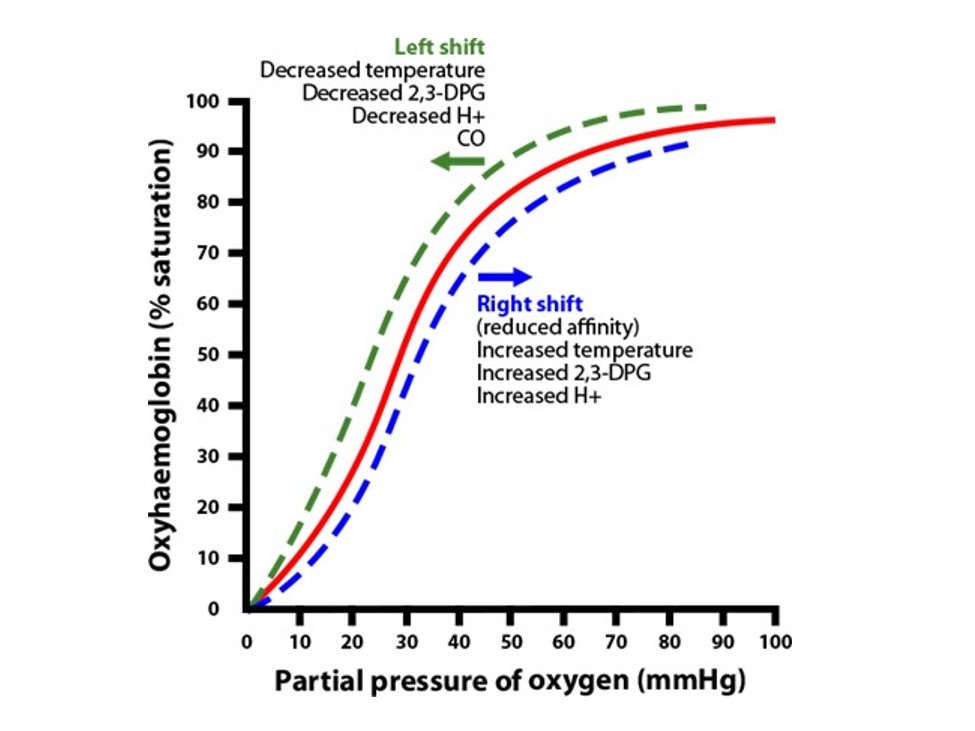
The following environments cause increased affinity of hemoglobin for O2, which shifts oxygen-hemoglobin dissociation curve to the left:
- Increased pH
- Decreased concentration of 2,3 BPG
- Decreased temperature
The following environments cause decreased affinity of hemoglobin for O2, which shifts the oxygen-hemoglobin dissociation curve to the right:
- Decreased pH
- Increased 2,3 BPG concentration
- Increased temperature.
A note on 2,3-BPG: 2,3-diphosphoglycerate (2,3-DPG), AKA, 2,3-biphosphoglycerate (2,3-BPG): This is a 3-carbon isomer of the glycolytic intermediate 1,3 biphosphoglyceric acid. This molecule stabilizes the deoxygenated form of hemoglobin by allosteric binding to hemoglobin and facilitates oxygen release at tissue sites.
So, think about a muscle exercising. As the muscle exercises, it consumes O2 and produces CO2. This leads to a lower tissue pH in that muscle. The exercising muscle consumes glucose, leading to glycolysis and production of 2,3-BPG. The temperature in the muscle rises with exercise/metabolism. All of this conspires to encourage the RBCs flowing into the muscle (and blood vessels dilate to allow more blood flow to active muscles, more later in a different block), where the environment facilitates release of O2 to the active muscle cells. Pretty amazing!!
And, if you thought about the fact that hemoglobin has an affinity for O2, then immediately one can guess that there are variant hemoglobins that have more (or less) affinity for O2. The classic one is fetal hemoglobin, which has a higher affinity for O2. Think about why a developing fetus would need a hemoglobin with a higher affinity than the mother’s hemoglobin…. Once you’ve answered that on your own, then think about a mechanism that would allow fetal hemoglobin to have a higher affinity for O2…. Clue: 2,3-BPG binds to hemoglobin, as we discussed above. If a hemoglobin had a higher or lower affinity for binding to 2,3-BPG, would that affect that hemoglobin’s oxygen-hemoglobin dissociation curve?
(Answer: Fetal hemoglobin has a higher affinity for O2 than normal hemoglobin, so its curve is shifted to the left relative to the dissociation curve for normal hemoglobin. Fetal hemoglobin has a higher affinity for O2 because it binds BPG less strongly. Because it binds to BPG less strongly, BPG is less able to enhance the ability of the fetal hemoglobin to release O2, compared to normal Hgb!)
Examples of Defects in Normal RBC structure and RBC Metabolic Pathways that Lead to Disease
High oxygen affinity hemoglobin:
Fetal hemoglobin, which is produced during the later part of fetal development and then is replaced by normal hemoglobin production after delivery, is a high oxygen affinity hemoglobin. This is normal in developing fetus, and is replaced by normal adult hemoglobin production starting around the time of delivery. More on this later…. (This isn’t a variant, but is an earlier form of hemoglobin present in developing fetus.)
High oxygen affinity hemoglobin variants (adult variants) do exist. Would you predict the p50 (pressure at which 50% of hemoglobin is saturated with oxygen) to be higher or lower with a high affinity hemoglobin—ie is the oxygen-hemoglobin curve shifted to the left or the right? What would you expect the hemoglobin to be (higher or lower) in a patient who has a high affinity hemoglobin variant?
(Answer: with a high affinity hemoglobin, it binds O2 more readily, so it doesn’t require as much oxygen tension to be 50% bound, so the pO2 needed to generate 50% of the hemoglobin bound to O2 would be lower. The oxygen-hgb curve is shifted to the left in this case. A patient with high affinity hemoglobin won’t release O2 to tissues as readily. This will cause increased hypoxia to be sensed by the kidneys, leading to increased epo (erythropoietin) production and, hence, increased RBC production. This will result in a higher RBC count and hemoglobin than normal, and this is generally labelled polycythemia. Hence, a patient with a high affinity hemoglobin will generally have a higher hgb/RBC count than normal. Note, Polycythemia, where the red cell count is too high, can lead to poor circulation/strokes in certain cases, and can be a significant health concern.
Ok, lastly, what is the most common cause of polycythemia (higher hgb/RBC) in patients? Discounting dehydration/fasting (which cause a spurious polycythemia on lab testing), the most common cause is hypoxia—often due to lung problems related to smoking—causing hypoxic drive in the kidney to produce more epo. (This is a foreshadowing of your respiratory lectures, here and how pulmonology ties into hematology!)
G6PD deficiency:
G6PD deficiency is a disorder where red blood cells are deficient in an enzyme called Glucose 6 phosphate dehydrogenase (G6PD). G6PD enzyme is an important part of the hexose monophosphate shunt. G6PD is specifically involved in catalyzing NADPP to form NADPH. The gene coding G6PD is located on the X chromosome. Hereditary G6PD deficiency is an X-linked disorder. Patients with this disorder lack G6PD, and this causes red blood cells to break down prematurely, particularly in settings of oxidative stress.
Recall from your FMR course that G6P (Glucose 6 phosphate) is involved in both the glycolytic pathway and in the production of NADPH. Glucose 6 phosphate dehydrogenase (G6PD) is necessary for the formation of NADPH. A deficiency of G6PD impairs the erythrocyte from producing NADPH. NADPH is involved in overcoming oxidant stress in cells. (As you can imagine, in a cell that carries oxygen, there is a lot of oxidant stress). Oxidant stress can occur from ingestion of certain drugs (eg rasburicase, dapsone, primaquine), foods (fava beans is the classic one), and infections. This oxidative stress leads to presence of denatured/precipitated hemoglobin (AKA Heinz bodies) in the Red blood cells— which can be seen using supravital stains such as new methylene blue. As these cells circulate, the spleen removes defective RBCs from circulation and recycles the cell components. This form of red cell destruction by the spleen (which is normal for old red blood cells and doesn’t result in anemia in adults without G6PD deficiency) is exacerbated in times of stress in patients with G-6PD deficiency, and such patients can develop a hemolytic anemia due to this intrinsic red blood cell defect because of increased destruction of RBCs by the spleen. This can appear as bite or blister cells on peripheral blood smear using standard H&E stain. The bite cell appearance may reflect partial opsonization from splenic macrophages or impaired staining of the denatured hemoglobin.
Take Home Points from this Session
- Red cell production occurs in the bone marrow supported by erythropoietin, leading to an anucleate circulating RBC.
- Heme synthesis is a multi-step enzymatic process with steps in the mitochondria and cytosol. Defects result in ineffective erythropoiesis, and some disorders.
- Hypoxia (anemia is an example of a blood disorder leading to hypoxia) is sensed in the kidney, leading to increased erythropoietin production.
- The RBC cytoskeletal membrane supports deformability. Defects in this impact RBC lifespan.
- The oxygen-hemoglobin dissociation curve can shift left or right based on changes in hgb oxygen affinity, influenced by factors in the RBC such as 2,3 BPG.
Authors and Contributors:
Corliss Newman, MD authored this course pack material to correspond to lecture slides covering Erythropoiesis and the Red blood Cell (RBC) available on Canvas. Kristi Rice, MD and Nicholas Burwick, MD provided editing assistance.
