10 Anticoagulants and Reversal Agents
Student Learning Objectives
- Describe the mechanism of action of thrombolytics and anticoagulants used clinically for venous thromboembolism.
- Implement the rationale behind laboratory testing to monitor and adjust the dose of heparin or warfarin during treatment.
- Discuss the mechanism of action and clinical uses for anticoagulant reversal agents.
Overview
You have learned about mechanics of hemostasis, including primary hemostasis (vasoconstriction and formation of the platelet plug via von Willebrand factor and platelet interactions) and secondary hemostasis (activation of the clotting cascade with the ultimate formation of a fibrin blood clot). We will now cover drugs that are used to treat patient who either have clotting or bleeding problems.
Please recall that when we talk about clotting factors, they are in inactive and active forms. For example, Factor X (ten) is generally referring to factor X not activated, while Xa refers to factor X that has been activated enzymatically and is participating in the clotting cascade.
Review: The following diagrams are a review of primary and secondary hemostasis, which you may find helpful as we now move on to discuss drugs that are used to treat bleeding and clotting disorders. 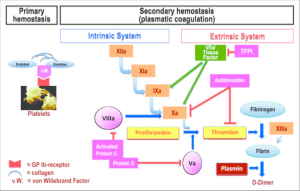
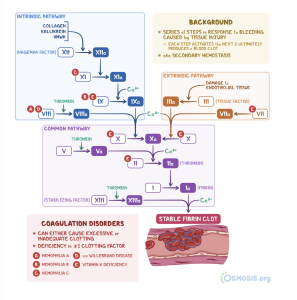
Fibrinolytics
Fibrinolytics are drugs that dissolve existing clots. Fibrinolytics have been used to dissolve large deep venous clots that are compromising blood return to the heart. You will be more familiar with fibrinolytics that are used to treat heart attack and stroke victims. You will likely hear more about fibrinolytic drugs in the cardiovascular and neurology blocks.
Anticoagulant Drugs (and their reversal agents)
Standard Anticoagulant drugs hinder the clotting cascade to prevent ongoing clotting and reduce the risks of a second blood clotting event in patients who have developed unwanted blood clots. For example, these drugs are classically used to treat patients with deep venous thrombosis. All these anticoagulants carry a risk for bleeding (since they inhibit the clotting cascade). Since patients on “blood thinners” (this is usually the slang term patients use for anticoagulants such as warfarin or direct thrombin inhibitors that prevent clot formation) sometimes have events where they are bleeding (think of a patient on a blood thinner who is in a car accident for example with major trauma), we have also developed drugs that reverse or counteract anticoagulants. These drugs are called “reversal agents”.
Drug: Heparins
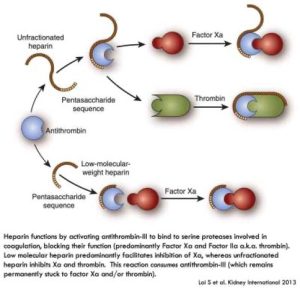
We’ll start with a class of drugs called Heparins. Heparins are drugs that work via antithrombin. All Heparins work by binding antithrombin (AT, also sometimes called antithrombin III or ATIII) and inducing a conformational change in AT such that it increases binding of AT to factor Xa and inhibits it from being active. Unfractionated heparin also works to inhibit thrombin (factor IIa) by binding AT and thrombin simultaneously. Heparins are broken down into 3 types:
- Unfractionated Heparin (classically referred to as “Heparin”). These are longer heparins (though they certainly include the pentasaccharide sequence, see diagram below) and these heparins work with antithrombin to inhibit factor Xa and activated thrombin (IIa).
- Low molecular weight Heparins, such as Enoxaparin. They have the key pentasaccharide part that binds to AT, but are shorter molecules than standard heparin. Because they are shorter molecules than heparin, they preferentially inhibit Xa, though there may be some IIa inhibition (although much less than unfractionated heparin).
- Fondaparinux: a pentasaccharide that binds to antithrombin and is too short to interact with antithrombin and thrombin simultaneously. Hence, Fondaparinux only inhibits Xa. Thrombin (IIa) is not inhibited by Fondaparinux.
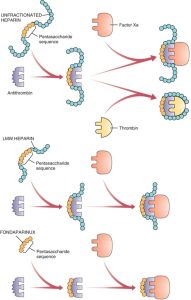
The following diagram depicts the differences in unfractionated heparin vs low molecular weight heparin. Fondaparinux is not shown, but one can see that Fondaparinux (which would be a shorter protein than low molecular weight heparin and would only have the pentasaccharide moiety) would only bind to antithrombin.
Here is another schematic you may find helpful:
Reversal agent for Heparin: Protamine Sulfate
Protamine sulfate is a drug that binds to Heparin (and by this we mean unfractionated heparin). It is mainly used to reverse the action of unfractionated heparin. It can be used to neutralize low molecular weight heparins such as enoxaparin, but is less effective at this. Protamine is a highly alkaline protein molecule. Interestingly, by itself it has a weak anticoagulant activity when administered alone. When protamine is given in the presence of heparin (in other words, given to a patient who already has been administered heparin), a stable salt is formed and the anticoagulant activity of both drugs is nullified. In the presence of low molecular weight heparin (LMWH, such as enoxaparin), protamine incompletely reverses the anti-factor Xa activity of LMWH. The half-life of protamine is short, and repeated doses may be necessary. Protamine is most commonly used in the setting of cardiac bypass surgery. During surgery, while a patient is on cardiac bypass, the patient is anticoagulated with heparin. After the vascular bypass is complete and as surgery is being wrapped up, protamine is given to reverse the anticoagulant effect of heparin.
Drug: Warfarin
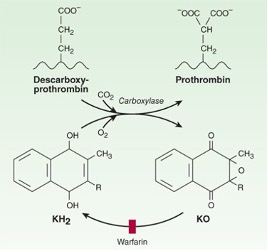
Clotting factors X, IX, VII, and II (think of the mnemonic “1972”) require gamma-carboxylation for activity. Clotting factor carboxylation is coupled to vitamin K oxidation. Once this occurs, Oxidized vitamin K is reduced by vitamin K epoxide reductase (VKORC1) back to the active reduced form of vitamin K. Hence, factors X, IX, VII, and II are known as vitamin K dependent clotting factors. (If you are pondering whether folks with vitamin K deficiency have a bleeding problem, the answer is yes!). Warfarin is a drug that works by binding to VKORC1 and inhibits recycling of oxidized vitamin K. It thus inhibits the synthesis of active clotting factors X, XI, VII, and II.
Warfarin used to be the mainstay of oral outpatient anticoagulation, before the advent of newer therapies. It is an inexpensive drug. It has previously been commonly used to treat deep venous thrombosis and pulmonary embolism. However, it has a few problems.
- In addition to inhibiting activation of X, IX, VII, and II, it also inhibits the activation of protein C and S, which are “anticoagulant” factors that slow down the clotting cascade. Hence, when warfarin is first started, it actually can have prothrombotic effects until the warfarin is fully therapeutic, which generally takes a few days. Because of this, when starting warfarin, one has to start another anticoagulant (generally a heparin drug) at the same time and continue that 2nd drug until the warfarin has been in a therapeutic range (INR of 2 to 3, see below) for a couple days. (After that, the second drug can be stopped.)
- Warfarin has a narrow therapeutic window and is affected by vitamin K intake in the patient’s diet. Because of this, its anticoagulation effects have to be monitored regularly—which is done by measuring the INR.
- There are many drugs that interact with warfarin and can cause the INR to go up or down. Antibiotics, for example, notoriously do this. So, if a patient is stable on warfarin and develops an infection and needs to start an antibiotic, the INR will need to be monitored closely and the warfarin dose will likely need to be adjusted both when starting and then when stopping the antibiotic.
Because of the above issues, warfarin has been largely replaced by the newer direct oral anticoagulant drugs (DOACs) such as rivaroxaban and dabigatran. However, warfarin is clearly more effective as an anticoagulant than DOACs in the setting of anticoagulation for mechanical heart valve replacements. One more note: Warfarin causes serious birth defects and cannot be used by pregnant individuals for anticoagulation.
Reversal of Warfarin is achieved by giving Vitamin K, 4-factor prothrombin complex concentrate, or fresh frozen plasma.
Drugs: Direct Oral Anticoagulants (Rivaroxaban and Dabigatran)
Direct oral anticoagulants (DOACs) directly inhibit clotting factors without working via antithrombin. These drugs have an advantage over warfarin in that they don’t require monitoring, and they are generally not affected by vitamin K in the patient’s diet.
Rivaroxaban binds to and directly inhibits factor Xa.
Dabigatran binds to and directly inhibits factor IIa (aka thrombin).
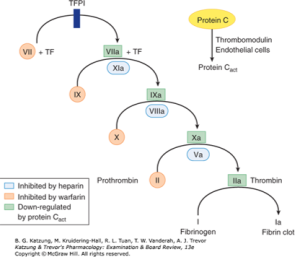
Drugs: DOAC Reversal Agents
When DOACs first came on the market, their convenience of administration and lack of monitoring was enjoyed, but the problem was what to do when patients came in with life-threatening bleeding. Unlike warfarin which could easily be reversed by giving clotting factors or Vitamin K, these newer drugs could not be reversed. Eventually reversal agents were developed.
Idarucizumab: This is a reversal agent against dabigatran. It is a monoclonal antibody fragment that binds to the thrombin-binding site of dabigatran with high affinity, such that dabigatran cannot bind thrombin (IIa). It is given intravenously.
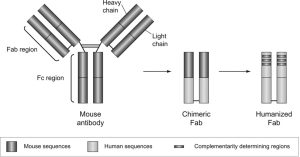
Figure 1. Development of idarucizumab. The fragment antigen-binding (Fab) region is composed of a light and heavy chain and contains the part of the antibody that binds to dabigatran. It also contains a constant region, which, when murine sequences are replaced with human ones, is called a chimeric Fab. The fragment constant (Fc) region interacts directly with the immune system; however, such nonspecific binding is avoided by removal of the Fc region.
Source: Idarucizumab The Antidote for Reversal of Dabigatran, John W. Eikelboom, Daniel J. Quinlan, Joanne van Ryn and Jeffrey I. Weitz Originally published22 Dec 2015https://doi.org/10.1161/CIRCULATIONAHA.115.019628Circulation. 2015;132:2412–2422
Andexanet alfa: This is a reversal agent that works as a modified recombinant factor Xa decoy. It binds and sequesters factor Xa inhibitors such as rivaroxaban such that they don’t bind and inactivate real Xa.
Drugs that dissolve clots: Fibrinolytics
Drug: Alteplase
Unlike the other drugs above, which inhibit clotting, Alteplase breaks down clots that have already formed. Hence, it is described as a “thrombolytic” drug. Alteplase is a recombinant form of tissue plasminogen activator (tPA). It binds fibrin in the thrombus. There, it converts plasminogen to plasmin. Plasmin then initiates local fibrinolysis (plasmin breaks down the fibrin that is holding the clot together).
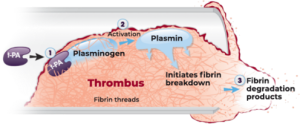
We have talked about disorders of clotting and drugs used to prevent clots and their reversal agents. We have also covered a drug used to break down clots.
We will now move on to briefly discuss a drug used to treat bleeding due to overly active fibrinolysis.
Most of the time, when we think of bleeding disorders, we think of disorders with lack of clotting factors such as hemophilia A (lack of factor VIII), hemophilia B (lack of factor IX) and Von Willebrand Disease (lack of VWF). However, other types of bleeding disorders, which are less common, are due to problems with the clot breakdown system such that clot breakdown is overactive (overly active fibrinolysis). There is a class of drugs that are antifibrinolytics, and the classic 2 drugs in this class are ε-aminocaprioic acid and tranexamic acid.
DRUGS THAT INHIBIT FIBRINOLYSIS:
Drugs: ε-Aminocaprioic acid and Tranexamic acid
Both drugs work to impair fibrinolysis. These drugs are lysine analogs that bind the lysine binding site on plasminogen. By binding at the lysine binding site on plasminogen, they prevent plasminogen being converted to active plasmin. Hence, they stabilize clots/prevent clot breakdown. As would be expected, their risk is thrombosis. Of note, Tranexamic acid is 3 times more potent than ε-Aminocaprioic acid.
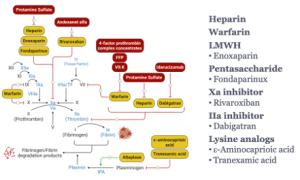
Dr. Wang’s summary slide from the corresponding lecture is an excellent summary of most of the drugs and reversal agents we have discussed and their sites of action in the clotting cascade.
Authors and Contributors:
Corliss Newman, MD wrote this chapter incorporating slide material provided by Edith Wang, PhD.