8 Weight Homeostasis and Disorders of Weight
Introduction
This chapter reviews the fundamentals of energy homeostasis including energy balance and the hormonal control of body weight. We will then go on to review the pathogenesis of obesity and cachexia.
Energy intake and energy expenditure are fundamental aspects of energy homeostasis, the process by which body fuel stored in the form of adipose tissue is held constant over long intervals. An imbalance in energy expenditure can lead to a change in body weight.
Body size is determined by a large number of known and unknown factors and falls naturally on a curve as all human traits do. It is also tightly regulated by the body in a process of homeostasis, such that energy intake and expenditure may not have the long-term effects that are apparent in the short term.
Some determinants of body size include genetics, stress, sleep, environmental effects, hormonal processes and disorders, medications, access to food, eating patterns, life stage and illness. Some of these will be addressed in this chapter.
Disorders in energy homeostasis can result in both increased body weight or decreased body weight.
Body Weight Regulation
Homeostasis refers to the ability of an organism to maintain a constant internal environment, thereby allowing survival over a wide range of external environmental conditions. Energy homeostasis is defined as the physiologic process that matches energy intake to expenditure over long time intervals to promote stability in the amount of fuel stored as fat. The regulation of energy balance is very complex. Energy intake and expenditure are regulated by the hypothalamus, the autonomic nervous system, the muscles, viscera, and adipose tissue.
The human body can store enormous amounts of energy in the form of triglycerides. Through well-established hormonal mechanisms, these energy stores can be rapidly mobilized for use by tissues when needed. Adipose tissue is unique in its ability to expand almost indefinitely when energy intake chronically exceeds energy utilization.
Triglycerides are stored within adipose cells (adipocytes) as a single lipid droplet that can grow until individual cells become very large. When individual adipocytes reach the limit of their ability to enlarge, new adipocytes are recruited from a pool of mesenchymal precursor cells. These processes are reversible, so that when energy utilization chronically exceeds energy intake, both the size and number of adipocytes decrease.
As energy balance changes, physiologic processes and body mass will change. Due to the nature of adipose tissue discussed above, 60-80% of body mass change will involve fluctuations in the fat mass. With negative energy balance, in addition to fat mass changes, metabolism will slow and muscle and, in some cases, bone mass will also be lost. This can lead to “sarcopenic obesity,” a condition correlated very strongly with cardiovascular disease and all-cause mortality.
Energy Expenditure
Energy balance is achieved when there is stability between energy intake (calories consumed) and energy expenditure (calories burned). Total daily energy expenditure (Figure 1) is composed of resting metabolic rate (RMR), thermic effect of exercise (TEF), and dietary thermogenesis. The RMR is the energy expenditure required for maintaining normal body functions and homeostasis (the minimal ongoing energy utilization required to sustain cardiac activity, respiration, neurological function, and all the metabolic activities of bodily tissues). The RMR is influenced by multiple factors including body size, age, growth, gender, hormonal systems (e.g. thyroid), infection, physical activity, medications, drugs and dietary deficiency. The RMR is proportional to body mass, in particular fat-free mass. The larger the body size, the higher the RMR will be (proportional to body mass). Resting metabolic rate accounts for 50-75% of total energy expenditure. TEF (thermic effect of food aka dietary thermogenesis) refers to the energy required to absorb, digest, and metabolize the food consumed and typically accounts for 8–10 % of daily energy expenditure. Diet-induced thermogenesis comprises energy expenditure by digestion, absorption, and storage of food and can be affected by the type of food ingested. For example, meals with high protein or carbohydrate content increase diet-induced thermogenesis more than do meals high in fat. The energy expended due to physical activity (EEact) accounts for energy that is expended in addition to the RMR and TEF, including voluntary exercise, shivering, postural control, and voluntary movement. When energy levels are in balance, body weight is stable. One effect of changes in energy balance may be a change in body weight and/or composition. The body also regulates RMR, appetite, sleep, and activity to keep body weight and energy balance stable.
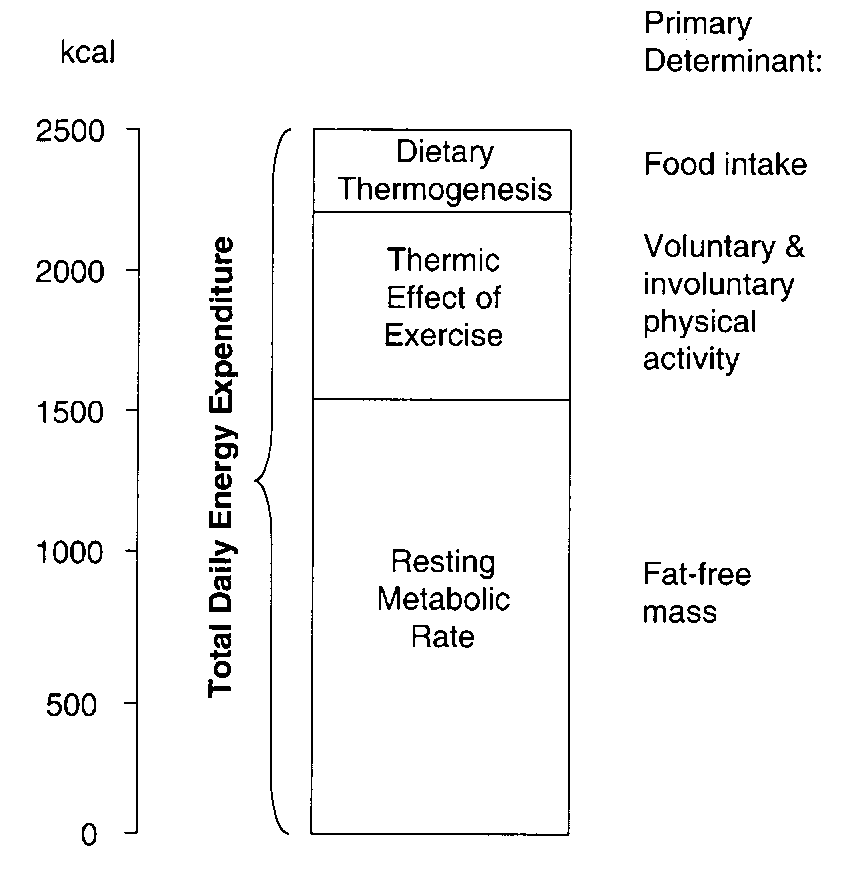
Resting energy expenditure is proportionate to an individual’s fat-free body mass. When an individual has deviated below baseline body fat content, these energy expenditure values are even lower than predicted. Conversely, when an individual has deviated above baseline body fat content, energy expenditure values are even higher than predicted for the increased fat-free mass. These exaggerated compensatory responses to weight loss and gain within an individual provide a strong adaptive force acting to restore the original body mass. This phenomenon explains the difficulty encountered by many individuals trying to lose weight through dieting.
In the absence of disease states or a history of weight cycling, RMR is roughly proportional to fat-free mass, meaning that larger bodies will require larger energy inputs to maintain function. Initially in the presence of negative energy balance, weight loss (of both fat and other tissue) will occur, but over time RMR will adjust causing weight to stabilize or increase without increase in intake.
CNS, Gut, Adipose Regulation of Energy Intake
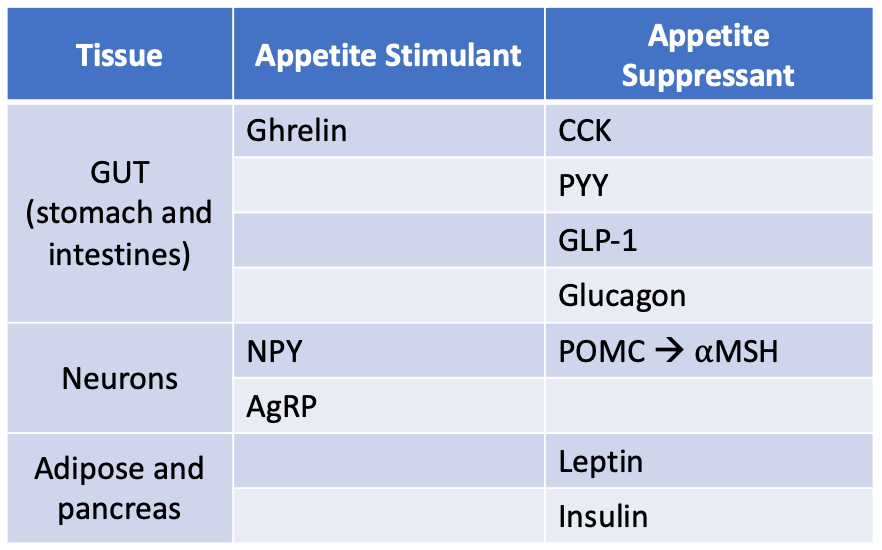
The regulation of energy intake and expenditure is a complex, multisystem process that involves the brain, the autonomic nervous system, the muscles, viscera and adipose tissue. As energy intake decreases, stored fuel sources decrease. When body fuel sources are depleted, signals from the gut and the adipose tissue act locally and centrally to stimulate food intake, reduce metabolic rate, and restore caloric balance. Table 1 provides a summary of the hormones involved in appetite regulation.
Central Regulation
The brainstem and the hypothalamus are the key brain regions that regulate energy homeostasis. The hypothalamus is the main brain center that handles energy homeostasis. The hypothalamus receives signals from the gut and adipose tissue, processes these signals and secretes neurotransmitters that regulate energy balance by affecting appetite. There are two main neuronal populations in the arcuate nucleus of the hypothalamus that control energy balance. One population of neurons makes neurotransmitters named neuropeptide Y (NPY) and agouti-related protein (AgRP). The other population of arcuate neurons critical to body weight regulation makes proopiomelanocortin (POMC) which is processed into the neurotransmitter alpha melanocyte-stimulating hormone (aMSH). NPY and AgRP stimulate appetite, while aMSH inhibits appetite (table 1). When the NPH/AgRP neuronal population is stimulated both NPY and AgRP increase and food intake increases. When the POMC neuronal population is activated, aMSH is released and food intake decreases.
The nucleus tractus solitarius (NTS) and the area postrema (AP) are located in the hindbrain and are key nuclei that sense and integrate peripheral nutritional signals leading to the regulation of feeding behavior. The AP mediates nausea and vomiting in response to sensor input. The NTS is the first projection site for the intestinal and gustatory afferents. When stimulated, food intake is decreased. The CGRP neurons in the parabrachial nucleus mediate aversive responses to agents that induce gut inflammation.
Gut Regulation
The presence of nutrients in the gut leads to secretion of acute onset hormonal signals that inform the body of nutritional status. The intestine and stomach secrete hormonal signals that feedback to the hypothalamus to control meal initiation or termination. Cholecystokinin (CCK) is secreted from the I-cells of the GI tract (duodenum) in response to lipid and protein intake. CCK is a neuropeptide and gut hormone that leads to release of digestive enzymes and bile from the pancreas and gallbladder, slows gastric emptying (thus increasing satiety) and signals to the hypothalamus to increase satiety. Peptide YY (PYY) is synthesized and released from enteroendocrine cells called L-cells found in the distal GI tract (ileum and colon). PYY concentration increases after a meal and decreases with fasting. PYY inhibits gastric motility (thus increasing satiety) and acts centrally to suppress activation of neurotransmitters NPY and AgRP. Fibroblast growth factor 19 (FGF19) is a gut hormone with pleiotropic effects. It is secreted by the small intestinal cells in response to feeding and inhibits food intake. Glucagon-like peptide 1 (GLP-1) is a hormone that will be discussed in more detail in the diabetes lecture (part 1). It is secreted by the L-cells of the distal intestine (ileum and colon) and delays gastric emptying and acts centrally to suppress AgRP/NPY. GLP-1 also controls blood glucose by enhancing glucose-dependent insulin release and decreasing hepatic glucose output (more on this in the diabetes chapter). Unlike PYY, GLP-1, and CCK which all lead to appetite suppression by several mechanisms, ghrelin is a gut-derived hormone that leads to appetite increase. Ghrelin is secreted by enteroendocrine cells of the GI tract (especially the stomach). Blood levels of ghrelin are highest before meals when the stomach is empty and return to lower levels after mealtime. Ghrelin activates NPY and AgRP neurons, leading to appetite stimulation. Ghrelin also acts in the anterior pituitary gland to increase growth hormone secretion.
Insulin is secreted by the pancreatic beta-cells in response to a rise in blood glucose and promotes glucose uptake into the tissues and inhibits food intake. Insulin leads to a reduction oin NPY and AgRP and binds POMC neuronal population thus inhibiting food intake. Insulin also regulates the action of leptin to decrease appetite (see below).
Adipose Regulation
Adipose tissue (AT) acts to store fuel in the form of lipids. It is also a highly active metabolic and endocrine organ. AT can expand in the form of hyperplasia (cell number increase) and hypertrophy (cell size increase). Not all forms of AT are created equal and only some adipose tissue leads to metabolic and endocrine complications. Visceral adipose tissue (compared to gluteal and subcutaneous adipose tissue) is more metabolically active, more insulin resistant, generates more free fatty acids, and secretes more harmful adipokines (cytokines that come from adipose tissue).
Leptin is a hormone secreted by white adipose tissue that informs the brain about the mass of adipose tissue. The higher the adipose tissue mass, the higher the levels of leptin. Leptin is not an acute signal. Instead, it functions to regulate long-term balance between energy intake and energy expenditure by informing the brain of adipose tissue mass. Different from the gut regulation discussed above, the signals from the fat mass are longer term signals that respond to changes in body fat mass. Leptin exerts feedback on the hypothalamus. With increasing levels of leptin, NPY and AgRP release is decreased. Leptin binds to POMC which leads to an increase in α-MSH, which binds to the melanocortin receptor, MC4R, and inhibits food intake.
There are rare monogenic causes of leptin deficiency. More commonly, obesity is associated with leptin resistance. Thus synthetic leptin has not been an impressive agent in the treatment of obesity.
Introduction to Obesity
Definitions
In this chapter, the word “obesity” is used to describe a medical condition characterized by excess body-fat mass and measured by the body mass index (BMI). Many people in larger bodies and their advocates reject the term “obesity” as well as the classification of body size as a disease.
The word “obesity” is derived from a Greek word meaning “to eat oneself sick,” which we know is not an accurate portrayal of people with bigger bodies. Bodies naturally come in a range of sizes. Though widely used, the BMI is not an accurate measure of adiposity (see below).
One drive behind designating obesity as a disease was the hope of destigmatizing body size and increasing engagement in healthy lifestyle. In fact, research shows that stigma has increased since the medicalization of body size, and that believing oneself to be overweight or obese has a negative impact on healthy lifestyle. Studies show that the phrase “morbidly obese” is particularly objectionable to both patients and parents. In fact, this designation is no longer used by the CDC and should not be used to describe patients.
Alternatives to “obese” when describing patients include “larger-bodied,” “at the higher end of the weight spectrum” or simply recording the actual weight or BMI. Some people are reclaiming the word “fat” as a neutral descriptor of body size. When approaching conversations about weight with patients, it is prudent to ask how the patient prefers to define their own body.
Regardless of terms used, there is a large body of evidence that demonstrates that the presence of obesity correlates strongly with multiple medical disorders.
Body-fat mass is best quantified with dual energy x-ray absorptiometry (DEXA) scan or MRI. These methodologies of quantifying fat are impractical and therefore alternative and more simplistic measures are used. These methods include measurement of the skin folds, measurement of circumference of different body parts, and the body mass index (BMI). All of these methods are inaccurate measurements of body fat mass and normal values can vary dramatically with age, race, and ethnicity. Of the non-invasive measures mentioned, the BMI is the best studied as a surrogate measure of body fat mass. The World Health Organization (WHO) defines obesity as a BMI of 30 kg/m2 or more (and overweight has a BMI greater than 25 kg/m2 and less than 30 kg/m2 (Table 2)).
|
BMI (kg/m2) |
Weight Classification |
|
<18.5 |
Underweight |
|
18.5 – 24.9 |
Normal range |
|
25.0 – 29.9 |
Overweight |
|
>30 |
Obesity |
Although the BMI is the most used tool to define and measure obesity, it is a biased assessment that is a flawed and overly simplistic measure of body-fat mass.
The BMI was developed by a mathematician in the 1800s who was attempting to define the “ideal human body” from an aesthetic and moral point of view. He noted that the measure obtained by taking the weight in kilograms (kg) divided by the height in meters (squared) created a typical standard deviation curve of the Northern European adult males he was studying. As such, it may be a useful measure of showing the distribution of body size in a given population. It is not a useful measure of determining an individual’s health or “ideal” body weight. The BMI does not account for variations in other components that make up body mass such as muscles and bone. The BMI and its correlations to adiposity, overall health and mortality is an even poorer measure in non-white populations, in the elderly, women, and the young. Furthermore, classifications of weight status using BMI often are not accurate assessments of health status. There are many studies that have found strong associations between BMI and mortality, many showing a U-shaped or J-shaped relation with the risk of mortality increasing when BMI goes over 35. Mortality risk also increases with low BMI as well. It is important to note that these studies can only show association, with many of the studies unable to control for important mediators (e.g. social determinants of health, weight stigma, weight cycling, insulin resistance, behaviors known to associate with chronic disease, stress). It is possible that BMI may be a marker or proxy for the many unmeasured variables. Despite the limitations, BMI levels do correlate with body fat presence and with future health risks.
Epidemiology of Obesity
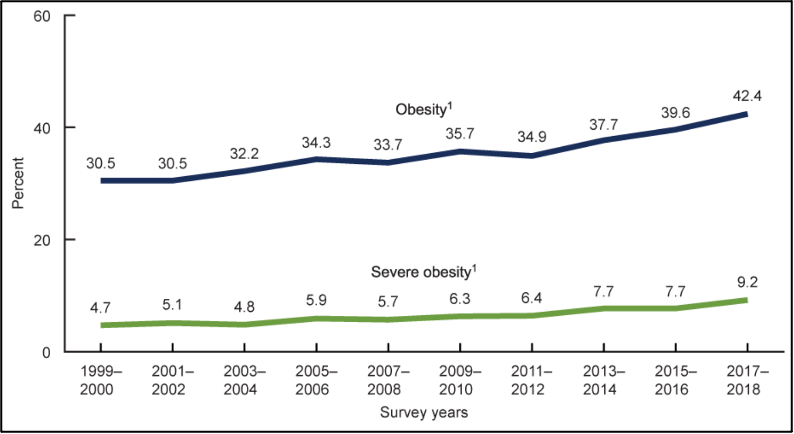
The presence of obesity is increasing in the United States. As patients with obesity experience higher rates of morbidity and mortality on average, this is a concern for the healthcare system. From 1999 – 2000 through 2017 – 2018, the US obesity prevalence increased from 30.5% to 42.4% (Figure 2).
Social Determinants of Obesity
According to WHO, the social determinants of health (SDH) are “the conditions in which people are born, grow, work, live, and age, and the set of forces and systems shaping the conditions of daily life.” Practically, SDH refers to social practices and conditions (such as lifestyles, living and work situations, neighborhood characteristics, poverty, pollution); socioeconomic status (income, education, and occupation); stressful circumstances; racial discrimination; and economic, political, and religious factors that affect (either positively or negatively) the health of individuals, groups, and communities.
SDH are being increasingly recognized as foundational issues that impact health.
Obesity prevalence in the United States varies by gender, age, and race/ethnicity. Obesity prevalence is higher in non-Hispanic Black and Hispanic adults compared with other racial and Hispanic origin groups. Obesity prevalence is higher in women, and in middle aged and older adults. The correlation between socioeconomic status and obesity is complex and varies by sex and race/ethnicity.
Social determinants of health correlate with body size and directly correlate with obesity-associated diseases, independent of body size. This raises the possibility that obesity is better understood as a marker of risk rather than a cause of risk. There is also downward socioeconomic pressure on larger bodied individuals. Weight stigma, including poorer healthcare may not only contribute to increased adiposity, but may also directly impact morbidity and mortality. Many studies of obesity related illness do not adequately control for SDH or weight stigma, confounding our understanding of risk and causation.
As above, BMI is also known to be less accurate in predicting adiposity and morbidity and mortality in diverse populations including Black, female and elderly populations.
Food insecurity (defined as experiencing stress or worry about having enough money to buy nutritious meals) is correlated with higher BMI. Food insecurity also correlates with other SDH associated with body size and morbidity. Mechanisms may involve adaptations of the body to unpredictable access to fuel such as lowering RMR, increasing insulin resistance, lowering leptin and increasing cortisol. This leads to decreased satiety when food is present, increased palatability of high fat and high sugar foods, and preferential deposition of adipose tissue for fuel shortage. Binge eating disorder is increased in populations without regular access to food as well, likely for similar physiologic and psychological reasons. This process may be exaggerated in caregivers who preferentially feed their children and are under higher levels of stress.
Several governmental programs are in place (primarily by the USDA and DHHS) in an attempt to address inequities in food access. The main programs you may hear about are: SNAP Food Benefits, the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), the National School Lunch and Breakfast Programs, Child and Adult Care Food Program (CACFP). Data has shown decreased rates of obesity in people enrolled in federal food assistance programs.
Although data has shown decreased rates of obesity in people enrolled in federal food assistance programs, many communities without equitable access to food object to using obesity rates as a measure of success of these programs, or as a reason to address food apartheid. Access to a variety of nourishing foods is a human right. Communities without this access often experience intersecting oppressions that may impact body size and health.
Medical Disorders Associated with Obesity
With increasing BMI, there is increased incidence of many diseases, particularly cardiometabolic disease. The relationship between BMI and overall mortality is curvilinear, with coronary artery disease accounting for most of the increase in death rate as the BMI starts to exceed 30 kg/m2. This increased risk for cardiometabolic complications is most clear at the highest end of the weight spectrum. There is strong data to show, however, that the BMI should not be used as a sole marker of health and that there are people with obesity who are metabolically healthy (shown by Tomiyama, et al 2016).
Commonly, the presence of excess adiposity is associated with dyslipidemia, elevated triglycerides, low HDL (good cholesterol) and increase in atherogenic VLDL (bad cholesterol). Metabolic dysfunction-associated steatotic liver disease is common in individuals with obesity. Visceral adiposity has long been associated with insulin resistance which leads to diabetes mellitus. This is likely related to elevated levels of inflammatory cytokines (adipokines) secreted from visceral adipose and low levels of adiponectin. The increase in total blood volume may result in hypertension, increased cardiac output and cardiac hypertrophy.
Adipose tissue is a complex metabolic and an important endocrine organ. As mentioned above, it is the primary site for energy storage in the form of lipids. It is important in normal physiology and obesity related complications. When adipose tissue is metabolically compromised, this leads to a proinflammatory state that leads to insulin resistance and increased free fatty acids (FFA) due to lipolysis. Chronically elevated FFA levels worsen insulin resistance. Dysfunctional fat cells also produce atherosclerotic-provoking adipokines increasing the risk for heart disease.
The mechanical forces of increased body weight can also cause hypoventilation, obstructive sleep apnea and reflux. Excess body weight also results in increased stress on joints, osteoarthritis and difficulty with activity. Polycystic ovarian syndrome (PCOS) which leads to irregular ovulation and impaired fertility is more common with obesity, as is decreased sperm count and low testosterone.
Research shows an increased risk of malignancy of 13 cancers in patients with elevated BMI.
While there is a strong correlation with morbidity and mortality at both ends of the BMI spectrum, determining the inflection point for increased mortality has been difficult despite many large metanalyses. The difficulty in establishing these correlations and, particularly, their relevance to an individual patient, is because of the numerous confounders of body size and mortality, the inherently flawed measure of BMI, the necessarily large time delay to analyzing huge population databases, and the improvement in cardiometabolic healthcare.
Importantly, these studies cannot account for weight stigma and anti-fat bias that contributes directly to metabolic disease as well as to reduced screenings, delayed diagnoses, lower quality healthcare, and less engagement in health-related behaviors. In addition, the designation of obesity as a disease and associated quality measures have led to increased stigma and decreased quality of care for patients with higher BMIs.
Pathogenesis of Obesity
The pathogenesis of obesity is incompletely understood. Assumptions in both lay and medical culture about “unhealthy” lifestyle choices contribute to nonscientific treatment strategies and to stigma. This section discusses several of the factors that are known to impact the pathogenesis of obesity.
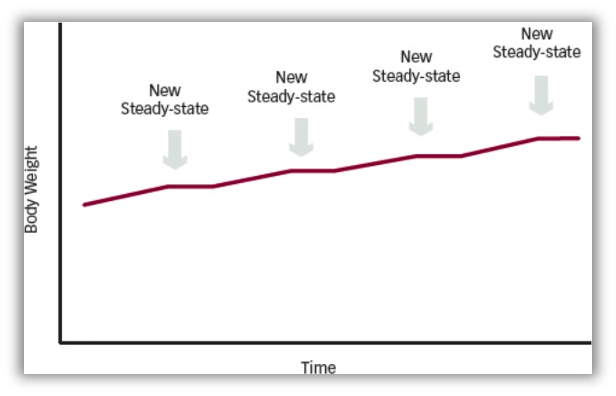
Obesity pathogenesis likely involves two related but distinct processes: (1) sustained positive energy balance and (2) resetting of the body weight set point at an increased value (Figure 3). The causes of these sustained positive energy imbalances are thought to be multifactorial – genetic, developmental, and environmental. Growing bodies of evidence support this explanation of obesity as a complex disorder of the energy homeostasis system (described earlier in this chapter), rather than simply arising from the passive accumulation of excess weight from overeating.
The stability of body weight over long periods of time and the clear genetic influence on body fat provide strong circumstantial evidence that adipose mass is physiologically regulated, rather than the consequence of poor habits or lack of will power. Numerous studies have shown that body fat mass is regulated to resist displacement either above or below its ordinary value, and that this regulation involves active modulation of both appetite and energy expenditure. The regulation of body weight is primarily geared towards preventing starvation, so the physiologic processes that resist weight loss are more robust than those that resist weight gain. This evolutionary trait in today’s current climate of relative caloric excess and highly palatable food choices may lead to a predisposition to weight gain. One possible contributor to the increased rates of obesity are the market and structural forces leading to both an inequitable and highly palatable food supply. The presence of toxins in food and water supplies (which are also inequitably distributed) may also affect neurohormonal regulation of energy and weight.
The hormonal changes that occur with any attempted weight loss demonstrate that it is exceedingly difficult to maintain a lower body weight. With any weight loss below the individual’s set point, changes occur to return the body to the set point. Energy expenditure decreases due to a drop in the resting metabolic rate (Figure 1). Additionally, the hormonal responses to weight loss lead to increased ghrelin and decreased leptin, PYY, CCK, and GLP-1. These hormonal changes stimulate NPY and AgRP and decrease POMC, which leads to increase in hunger signals. These hormonal changes persist for up to 42 weeks after initial weight loss. Over time, with “weight cycling” higher body weight set-points are defended. The mechanism for this is unclear but may relate to evolutionary adaptations to periods of famine.
Hypothalamic damage can also lead to obesity. The pathogenesis of obesity from hypothalamic insult lies in injury to the melanocortin system. When individuals are unable to produce -MSH they have hyperphagia due to lack of appetite suppression. In addition to trauma, diets high in fat promote inflammation and neuronal injury in the hypothalamus leading to obesity as well.
Rarely, obesity is caused by complete lack of energy regulation and occurs in those who have suffered hypothalamic injury or those with various genetic abnormalities (described below). Generally, single gene mutations leading to obesity are exceedingly rare and represent a small minority of the many causes of obesity. Although rare, understanding these conditions can help clarify the role of hormones in regulation of body weight.
Leptin Deficiency
Leptin is one of the key hormones involved in energy regulation. It is released from adipose tissue in proportion to fat stores and acts to suppress appetite. Leptin signals at the level of the hypothalamus to modulate signaling of the sympathetic nervous system, acting to increase lipolysis and inhibit lipogenesis. While this does lead to a decrease in fat stores, leptin’s role is primarily an adaptation to low energy intake, acting to mobilize fat stores in times of fasting and starvation, rather than prevention of excess energy intake. In addition to its role in energy balance, leptin is required for normal functioning of the reproductive axis.
Leptin deficiency is rare, with only a few dozen cases reported in the medical literature. Deficiency of leptin results in obesity due to extreme hunger. Patients with leptin deficiency present with early-onset, severe obesity. They show behavior and physiologic signs of starvation, with hyperphagia. They also develop hypogonadotropic hypogonadism. Leptin is an available therapy for these patients and corrects all abnormalities.
After the discovery of leptin, it was hoped that this could be used as a therapeutic treatment for obesity. By giving leptin to patients with obesity, it was hoped this would increase lipolysis and stimulate weight loss. However, most patients with obesity have high leptin levels, indicating common obesity is an example of leptin resistance, rather than leptin deficiency.
POMC deficiency
Absence of POMC neurons is rare, with only 50 reported cases. These patients are unable to produce α-MSH, which prevents appetite suppression. They have severe obesity due to hyperphagia. POMC is also a precursor for ACTH, which is required for normal adrenal function. Without ACTH production, these patients also have adrenal insufficiency. Patients tend to have red hair and pale skin due to absence of α-MSH. In 2022, a new medication was described (MC4R agonist – setmelanotide) that is effective in treating POMC deficiency and MC4R mutation (see below).
MC4R Mutations
MC4R is the receptor for α-MSH (and is inhibited by AgRP). This is the most common genetic cause of obesity, thought to be responsible for 2.5% of all cases of obesity. There is no specific phenotype for these patients other than increased body weight. Patients with MC4R mutation can be successfully treatment with GLP1-receptor agonists.
Treatment of Obesity
Despite a rising prevalence of, and focus on, obesity, effective strategies for the long-term suppression of adiposity are not yet available. It is important that physicians not oversimplify the process by recommending a “calorie in- calorie out” approach to weight loss.
Many excellent studies have shown that social, dietary and exercise interventions have large positive impacts on morbidity and mortality independent of impact on BMI. For this reason, initial behavioral coaching should be individualized and focus on improving engagement in general medical care, sustainable dietary changes, increased movement, social engagement, and mental health.
A comprehensive approach to weight loss involves dieticians, physicians, psychologists and support groups. Importantly, studies investigating these approaches have failed to control for the components of the intervention that may improve health without affecting body weight such as social, psychological and medical support and care.
Current options for weight loss include lifestyle intervention, pharmacotherapy, and bariatric surgery. Lifestyle intervention, including behavior modification, diet, and exercise, is the fundamental component of all obesity management.
Because weight stigma can reduce engagement in medical care and weight management often occupies large amounts of medical visits, care must be taken to assure that patients with obesity have access to the same screenings and evidence-based treatments for the diseases that occur with higher frequency such as type 2 diabetes, hypertension, sleep apnea, hyperlipidemia, and osteoarthritis.
Lifestyle Interventions
In the short term, negative energy balance results in improvement of many physiologic measures of health including blood pressure, insulin resistance (which leads to diabetes mellitus) and inflammatory markers, as well as reduction in fatty deposits in the liver. For this reason, many of these conditions have been termed “weight responsive illnesses.” However, achieving long-term (>5-10 years) sustained weight loss is exceedingly difficult due to the physiologic adaptations we have previously discussed.
In the simplest terms, body weight loss is achieved by sustained negative energy balance. The majority of Americans who are overweight (roughly 80% of those individuals are trying to lose weight) choose dieting as their sole lifestyle alteration. The unregulated non-medical diet industry is a multi-billion dollar industry and the overwhelming majority of Americans of all sizes report dieting at some point in their lives. Research does not favor any weight-loss diet as more efficacious than another. Food should remain palatable and enjoyable.
Although patients are frequently blamed, and blame themselves, for “falling off the wagon” over time and expanding their food volume and variety despite intentions, it is more likely that the counter-regulatory processes described above are responsible for return to higher caloric intake and return of weight. It is very important not to use one’s personal experience with diet and weight to advise patients, given multifactorial determinants of diet, energy balance and weight.
Exercise and increasing fitness are important determinants of health independent of impact on adiposity and are therefore an important component of medical care for all patients. Exercise has a role, combined with dietary restriction, in achieving or maintaining weight loss, but, in general, exercise alone is not effective in causing significant weight loss. Exercise is more effective in preventing weight regain after weight loss. Some data suggest that prolonged, low-intensity activity may be more effective in promoting weight loss compared to brief, high-intensity exercise. For many people, acute or prolonged exercise stimulates compensatory increases in appetite that can minimize weight loss. However, most subjects improve lean mass, lose some fat mass and improve fitness, all of which makes the effort worthwhile related to health outcomes. In contrast, activity of some kind (30-60min/day) is an essential, voluntary way to increase energy expenditure in subjects who have lost weight by dieting and now have reduced RMR (involuntary energy expenditure), meaning that exercise can help maintain weight loss. It should be noted that several components of the weight loss process make exercise difficult. Reduced RMR, restricted energy intake and muscle mass loss contribute to a reduced drive for nonessential movement. Patients or doctors may judge this physiologic drive to sedentariness as ”laziness” or ”lack of enjoyment” of exercise.
Physical activity and exercise may be difficult for patients with obesity. In addition to reduced energy availability and muscle loss that occurs with dietary restriction, some limitations may include access to safe and non-stigmatizing spaces, equipment and clothing, skin irritation, and un- or undertreated cardiorespiratory, autoimmune or joint disease. Furthermore, SDH may impact an individual’s ability to have time or a safe place to exercise.
Studies show that leisure time physical activities correlate with improved health even if they are not “exercise.” It’s important to start by asking patients about current levels of movement and past positive or negative experiences with exercise, before creating an individualized plan for increased movement. Many patients with obesity report that physicians assume they are not exercising based on their body weight.
Medications for Weight Management
In the 1960s, the use of psychostimulants for weight loss resulted in addiction and hypertensive emergencies. In the 1980s, the distribution of “fen-phen” (dexfenfluramine-phentermine) led to over 100 cases of valvular heart disease. Even as recently as 2006, the drug rimonabant (a cannabinoid antagonist that prevents “the munchies”) was approved in Europe only to be withdrawn 2 years later after post-marketing analysis revealed a twofold increase in psychiatric disorders including suicidality. Finally, the serotonin-norepinephrine reuptake inhibitor sibutramine, in use for over 10 years, was removed from the market in 2010 due to its association with an increased incidence of cardiovascular disease and stroke. As recently as February 2020, lorcaserin, a selective serotonin receptor agonist that decreases food intake in humans, was voluntarily withdrawn from the market after a large clinic trial found an increased risk of cancer in patients taking the medication.
Despite this history, guidelines recommend people are considered for medical treatment of obesity if the BMI is ≥30 kg/m2, or between 27 – 29.9 kg/m2 with comorbidities, who have not met weight loss goals despite lifestyle intervention. In all cases, weight loss medications do show weight loss compared to placebo, although the overall effect is small. There is a paucity of long-term data, however. There are currently six medications approved for long term use in the US. Current weight loss medications are included here for clinical familiarity but will not be tested on. Three of these medications belong to the class of GLP1 receptor agonists, which will be covered in the diabetes chapter. Given the history of pharmacotherapy for obesity, the limited weight loss achievable with most medications, and the lack of long-term data, we recommend that the choice to treat be balanced with the likelihood that a particular patient’s weight is clearly contributing to otherwise untreatable disease prior to initiating medications.
Orlistat inhibits pancreatic lipases, which limits fat absorption. This results in fecal fat excretion and inhibits absorption of fat calories. In a large meta-analysis, patients on orlistat lost 5-10 kg compared to 3-6 kg for the placebo group. Weight loss was maintained for up to 36 months while continuing to take the medication. Orlistat does improve both glucose and lipids. The side effects are primarily gastrointestinal in nature including cramps, flatus, fecal incontinence and oily diarrhea. These can be minimized by adhering to a low-fat diet. There have been 13 cases of severe liver injury described with orlistat use.
Liraglutide, an injectable GLP-1 receptor agonist used in the treatment of type 2 diabetes, can also cause weight loss. This is likely due to its effect to decrease appetite and increase satiety by inhibiting gastric emptying. In diabetes trials, it was noted that patients on liraglutide lost between 2-4 kg compared to placebo. In weight loss trials, with higher doses of liraglutide patients lost an average of 8.0 kg, compared to 2.6 kg for the placebo group. The main side effect is nausea, affecting nearly 50% of patients and should not be prescribed in a patient with a history of pancreatitis. Medications in this class are contraindicated in people with a personal or family history of medullary thyroid cancer (MTC) or multiple endocrine neoplasia type 2 (MEN2) due to rodent studies showing increased risk for thyroid C-cell tumors.
For the past 17 years, Orlistat was only one medication approved for teenagers. In December of 2020 the FDA has approved anti-obesity medications for teens; the pediatric version of liraglutide is approved for ages 12 and up.
Semaglutide, a GLP-1 receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control and has weight loss effects.
Tirzepatide is a combined GLP1/GIP receptor agonist. This medication is used for the treatment of diabetes and for the treatment of obesity. The medication causes appetite suppression.
There are several combination drugs available to treat obesity as well. Phentermine-topiramate results in a 10 kg weight loss over the first year of use, compared to 2 kg for the placebo group. It combines an adrenergic agonist with a neurostimulator and leads to weight loss through appetite suppression. The most common side effects of this medication are dry mouth, depression and difficulty with focus. It is not recommended for patients with cardiovascular disease, and due to risk of seizures from topiramate withdrawal, the medication cannot be stopped abruptly. This is the most effective obesity medication available, although as with others, long-term studies are lacking.
The final weight loss medication approved for long term use is a combination of bupropion-naltrexone. Bupropion as monotherapy is used for depression and the prevention of weight gain in smoking cessation. Naltrexone is an opioid-receptor antagonist used in treatment of opioid dependence. The medication causes weight loss by suppressing appetite. In clinical trials, patients on bupropion-naltrexone lost 5% of their body weight compared to 1% in the placebo group, but only 50% of patients completed a full year of treatment. Nausea, headache and constipation are the most common side effects. There is currently no long-term cardiovascular safety data regarding this medication. In clinical trials patients on bupropion-naltrexone had higher blood pressure and heart rate compared to the control group. As well, bupropion has been associated with suicidal thoughts in young adults, so providers must be aware of this risk. Its use is contraindicated in patients with seizure disorders, history of eating disorders or chronic opioid use.
Metabolic and Bariatric Surgery (MBS)
MBS has been shown to be the most effective and durable treatment for severe obesity. Given the poor track record of diets alone inducing lasting weight loss, there is a groundswell of interest in surgical interventions for lasting treatment of obesity and its related metabolic co-morbidities. Over 252,000 MBS procedures were performed in the US in 2018. According to the American Society for Metabolic and Bariatric Surgery, 61.4% patients undergo the vertical sleeve gastrectomy, 17% undergo the roux-en-y gastric bypass, 15.4% have a revision surgery and the rest consists of either adjustable gastric band, the biliopancreatic diversion with duodenal switch or other type of bariatric surgery. Current guidelines suggest consideration of MBS for patients with BMI> 35 kg/m2 regardless of the presence or severity of comorbidities associated with obesity, or BMI> 30 kg/m2 with metabolic disease associated with obesity. Studies show that surgery can reduce a persons’ risk of premature death by 30-40%. Despite strong evidence behind bariatric or metabolic surgery, there are many barriers in obesity treatment as currently over 15 million Americans qualify for surgery, but only about 1% have undergone surgery. Obesity care and surgical treatment is also not covered by many health insurance plans.
An interdisciplinary team approach is required to proceed with surgical treatment of obesity. The best results occur with increased post-weight loss activity and support by friends, family or health care providers. In addition, patients need life-long monitoring for complications and dietary supplements to prevent or correct nutrient deficiencies. Obesity is a chronic, lifelong disease and weight regain should not be viewed as a failure. No single operation, diet or medication by itself offers a permanent cure.
There are two major types of MBS (vertical sleeve gastrectomy and Roux-en-Y gastric bypass) and the amount of weight loss seen with MBS varies based on the procedure. The restrictive surgeries include adjustable gastric banding (rarely used) and vertical band gastroplasty. These limit the available space in the stomach and thus limit meal size. The adjustable gastric band and its predecessor, the open vertical band gastroplasty (or stomach stapling) procedures have fallen out of favor due to poor weight loss, high reoperation, and complication rates. Currently, the most common procedure in the US is the vertical sleeve gastrectomy. The sleeve gastrectomy is an irreversible operation where over 85% of the stomach is permanently removed, creating a tube or “sleeve” shaped stomach.
Malabsorptive surgeries alter the flow of nutrients and biliopancreatic enzymes in the GI tract due to the surgical alteration of the intestines. This decreases the functional length and area of absorption of the intestines. The most common procedure world-wide is the Roux-en-Y gastric bypass procedure, which combines a restrictive procedure, reducing the stomach to a 30 ml pouch, and malabsorptive procedure, attaching the stomach to the jejunum and bypassing the lower stomach and duodenum. The biliopancreatic diversion with duodenal switch is the most effective bariatric operation and offers greater magnitude of diabetes remission and weight loss, but due to the significant malabsorption this is not commonly performed. Figure 4 demonstrates the three most used bariatric surgery procedures.
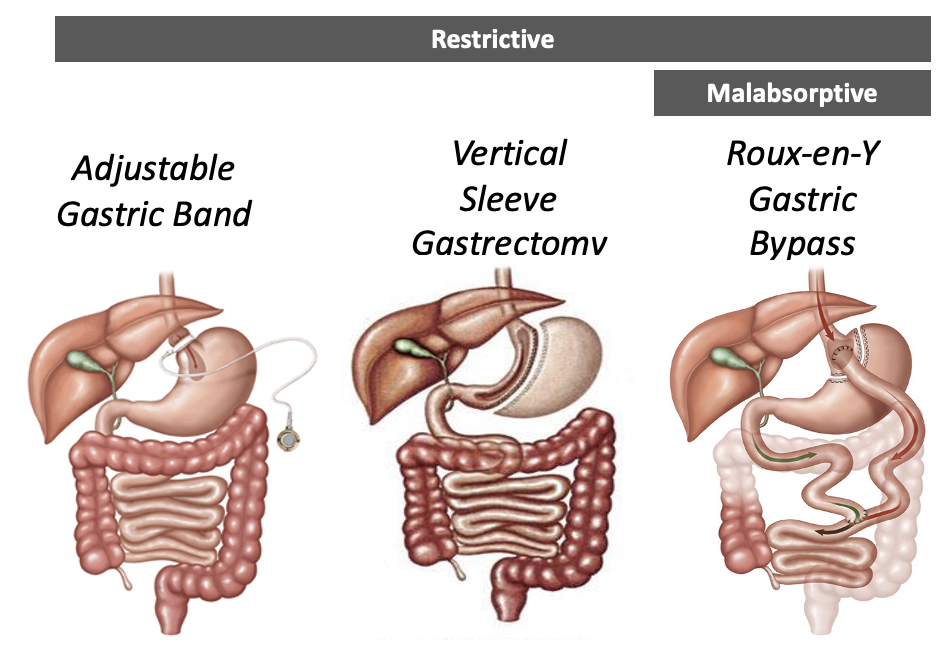
All these surgeries require a high level of technical skill and clinical expertise in choosing patients appropriate for the procedure, as well as managing postoperative care. Weight loss is greater with the malabsorptive procedures than the restrictive procedures (Table 3). Weight loss of greater than 50% of excess body weight has proved feasible with current surgical techniques and is associated with short-term (1/2 to 2 year) improvement in type 2 diabetes, hyperlipidemia, hypertension and obstructive sleep apnea. The maximum weight loss is reached at postoperative year 2. Weight regain is common after bariatric surgery. After roux-en-y gastric bypass, 93% of patients maintained at least a 10% weight loss from baseline, 70% maintained at least a 20% weight loss, and only 40% maintained at least a 30% weight loss after 12 years. Weight regain can be frustrating for patients and can lead to return of obesity-related comorbidities. Factors to prevent weight regain are close follow up from the bariatric surgery team and regular physical activity.
|
Effect of Procedure |
Vertical Sleeve Gastrectomy |
Roux-en-Y Gastric Bypass |
|
Mechanism of weight loss |
Restrictive |
Restrictive, Malabsorptive |
|
Reversible? |
No |
No |
|
Typical Maximum Weight Loss |
25-30% |
20-40% |
|
Type 2 Diabetes Resolution/Improvement |
40-50% |
65-85% |
|
Hypertension Resolution |
50% |
80% |
|
Average Costs |
$17,000-$20,000 |
$17,000-$28,000 |
|
Mortality |
0.2-0.5% |
0.5-0.9% |
Among patients with obesity, bariatric surgery was associated with longer life expectancy than usual care. Bariatric surgery leads to improvements in glycemic control and often leads to complete remission of type 2 diabetes. Additionally, bariatric surgery leads to improvement in cardiovascular health, resolution of sleep apnea, and relief of joint pain. In addition to improvements in the co-morbidities associated with obesity, all bariatric surgeries alter neurohormonal effects on energy balance. These changes can alter a patient’s appetite regulation, with an increase in appetite suppressing hormones PYY and GLP-1, and a decrease in the appetite stimulating hormone ghrelin (Table 4).
|
Gut Peptide |
Basal levels |
Post-prandial levels |
|
GLP-1 |
Unchanged |
Increased |
|
PYY and CCK |
Unchanged |
Increased |
|
Ghrelin |
Conflicting data |
Decreased |
It is important to be aware of how the altered anatomy of bariatric surgery will have implications on a patient’s routine care. In addition to complications from the surgery itself, the altered anatomy with the malabsorptive procedures can lead to multiple nutritional deficiencies. The absorption of calcium, iron, fat soluble, and B-complex vitamins are all decreased and require lifelong supplementation. Malabsorption can also lead to protein deficiency. Post-operative care following bariatric surgery requires routine follow up with monitoring for possible complications (Table 5).
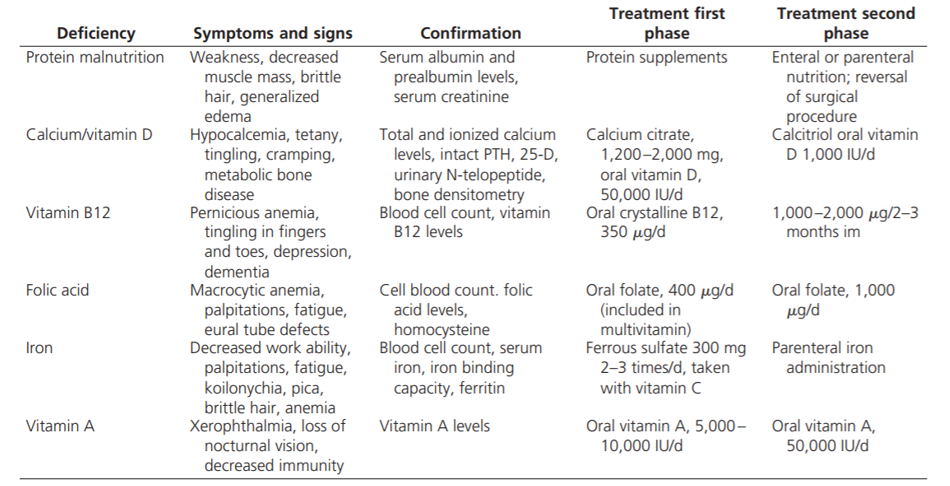
Bariatric surgery does not alter the necessity of lifestyle modification, as it still requires nutritional change and understanding of anatomy changes. Best results occur with increased post-weight loss activity, having a support system, and routine follow up. There will be gradual weight gain over time, as obesity is a chronic disease. People who have had bariatric surgery need life-long monitoring for complications and dietary supplements to prevent or correct nutrient deficiencies, as well as continued support for their mental health. This includes 1) commitment to a modified lifestyle, 2) more activity and exercise, and 3) consuming fewer calories.
Underweight
Underweight is defined as 18.5 kg/m2 and severely underweight is defined as 16.5 kg/m2. When considering the pathogenesis of underweight, the basics of energy homeostasis can be relied upon. Underweight is due to decreased energy intake or increased energy expenditure. Essentially, an imbalance in energy needs versus expenditure will lead to a deviation in body size.
Cachexia is a multifactorial syndrome characterized by severe body weight, fat and muscle loss and increased protein catabolism due to an underlying disease. In the setting of cachexia, appetite decreases (despite the presence of a starvation state), the metabolic rate is elevated, and lean body tissues are used as fuel instead of fat stores. These processes represent a disordered neuroendocrine response to the body’s nutritional status. Inflammatory cytokines are increased in chronic disease, and it is these cytokines that play a major role in the pathogenesis of cachexia. Inflammatory cytokines increase melanocortin signaling by inhibiting AgRP neurons and stimulating POMC neurons, leading to appetite suppression and weight loss.
On the other hand, an appropriate or physiologic response to calorie restriction include an increase in appetite, decrease in metabolic rate, and utilization of fat for fuel (with preservation of the lean body tissues).
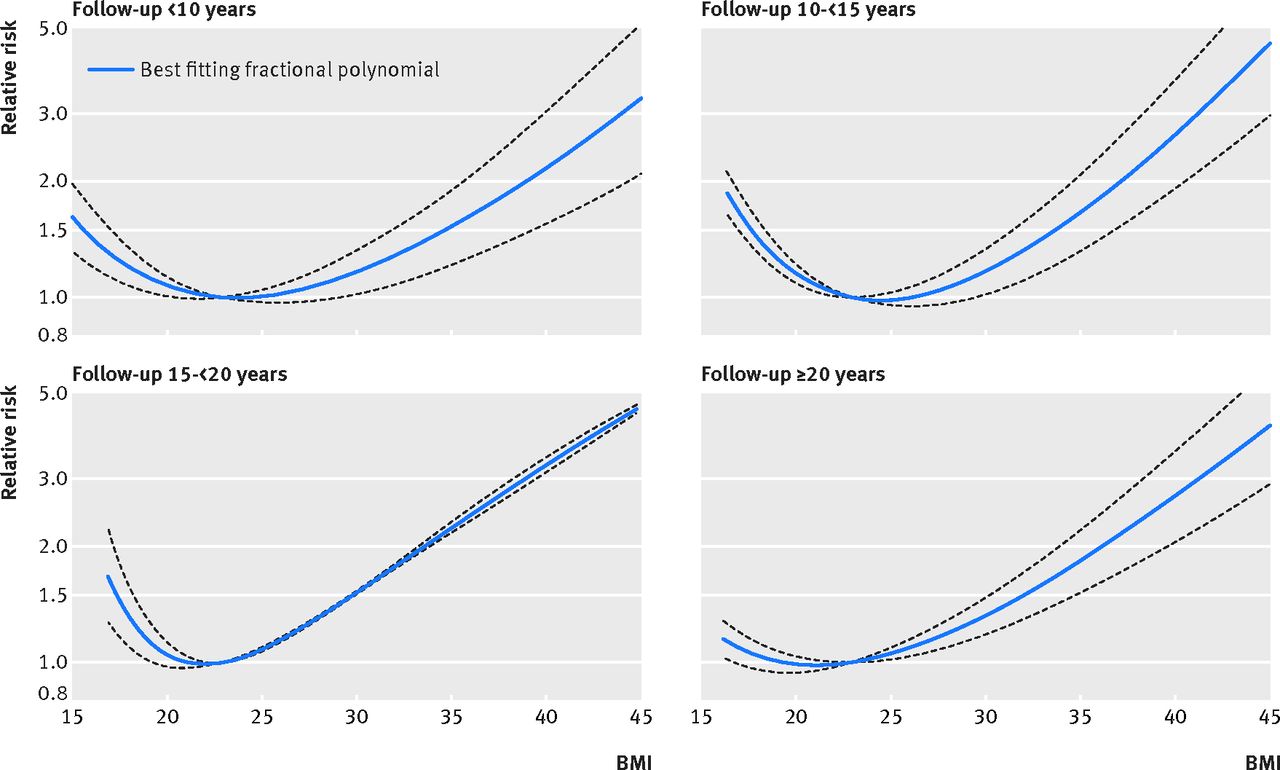
Similar to the presence of obesity, underweight or low BMI, has an association with increased mortality (Figure 5). The cause for increased mortality in those who are underweight is not understood. Several studies have demonstrated that there is an increased mortality risk driven by causes other than cancer, cardiovascular disease, respiratory disease and smoking status. External causes of death (e.g. suicides, accidents) are more common in underweight individuals than in other BMI categories. The reason for this is unknown, but it could be speculated that underweight may lead to impaired healing.
The pathogenesis of underweight is multifactorial and typically seen as a symptom of an underlying medical condition such chronic illness, inability to eat, or a social condition such as food insecurity. The situations that contribute to low BMI are vast. Major causes that contribute to low BMI are listed in Table 6.
| Major Causes |
| Malignancy (pancreatic cancer, lung cancer, GI malignancies, |
| Nonmalignant GI diseases (GERD, celiac disease, IBD) |
| Psychiatric disorders (depression, dementia, delirium, eating disorders) |
| Endocrinopathies (hyperthyroidism, diabetes, adrenal insufficiency) |
| Infectious diseases (HIV, tuberculosis, chronic fungal, bacterial, or helminth infection) |
| Advanced chronic disease (heart failure, renal failure, pulmonary cachexia) |
| Neurologic diseases (stroke, dementia, Parkinson’s) |
| Medications/substances |
| Rheumatological diseases (severe RA, giant cell vasculitis) |
| Chronic vigorous exercise |
| Medications/Substances Associated with weight loss |
| Alcohol |
| Cocaine |
| Amphetamines |
| Drug withdrawal syndromes |
| Tobacco |
| Prescription drugs (anti-seizure medications, diabetes medications, thyroid medications) |
| Herbal/non-prescription drugs (caffeine, nicotine, long list of herbal medications) |
