3 The Thyroid Gland
Introduction
Thyroid physiology illustrates several core concepts in endocrinology, such as the multiple effects of a single hormone and the simple negative feedback loop. Thyroid disorders are quite common, and thus, all providers regardless of specialty should be able to recognize and manage them. In this section, we will cover thyroid hormone synthesis, secretion, regulation, and action. In addition, you will become familiar with basic thyroid function tests, as well as the clinical presentations and management of hypothyroidism, hyperthyroidism, and thyroid nodules.
Thyroid gland development, anatomy, and histology
Anatomy
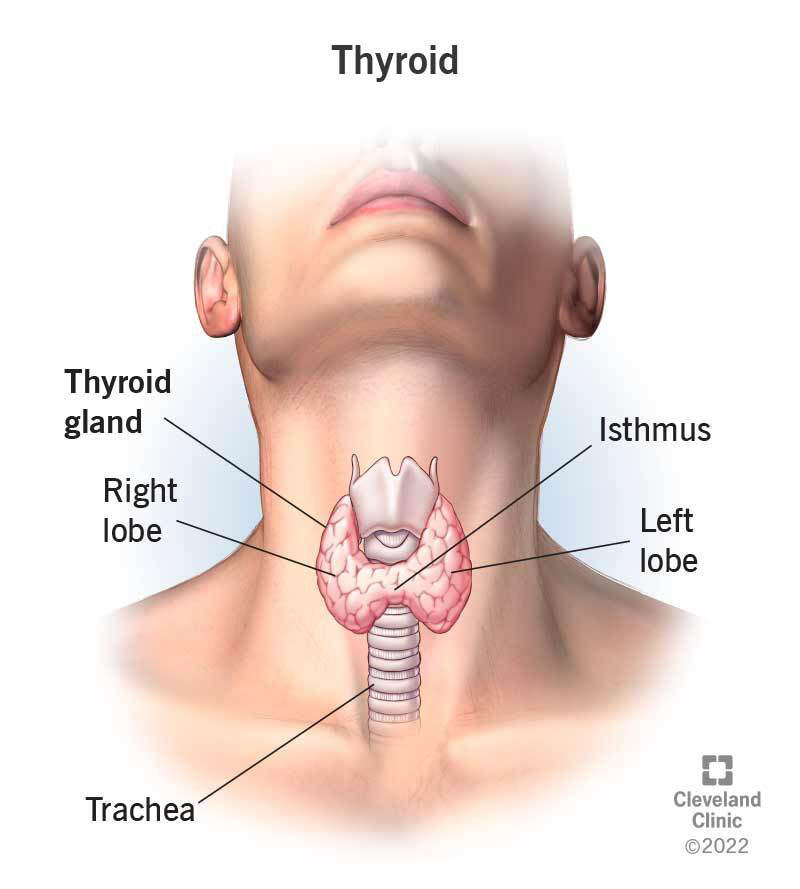
The adult thyroid resembles a butterfly (Fig 1), with a narrow isthmus lying across the trachea anteriorly and joining two 5 x 2 cm lobes. Two pairs of parathyroid glands (that control calcium levels) lie on the posterior surface of the lateral thyroid lobes, and the recurrent laryngeal nerves (that are important for vocal chord function) (Fig 2) run medial to them in the cleft between the trachea and esophagus. Thyroid surgeons meticulously avoid these structures to prevent the complications of iatrogenic hypoparathyroidism with hypocalcemia (due to inadvertent removal of the parathyroid glands) and vocal cord paralysis (due to injury to the recurrent laryngeal nerve). Thyroid gland anatomy and embryology will be covered in more detail in a later course.

Histology
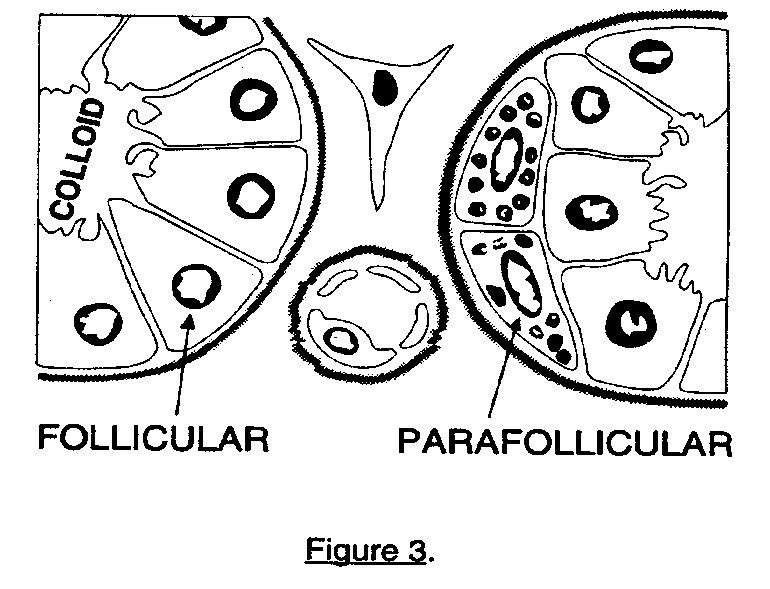
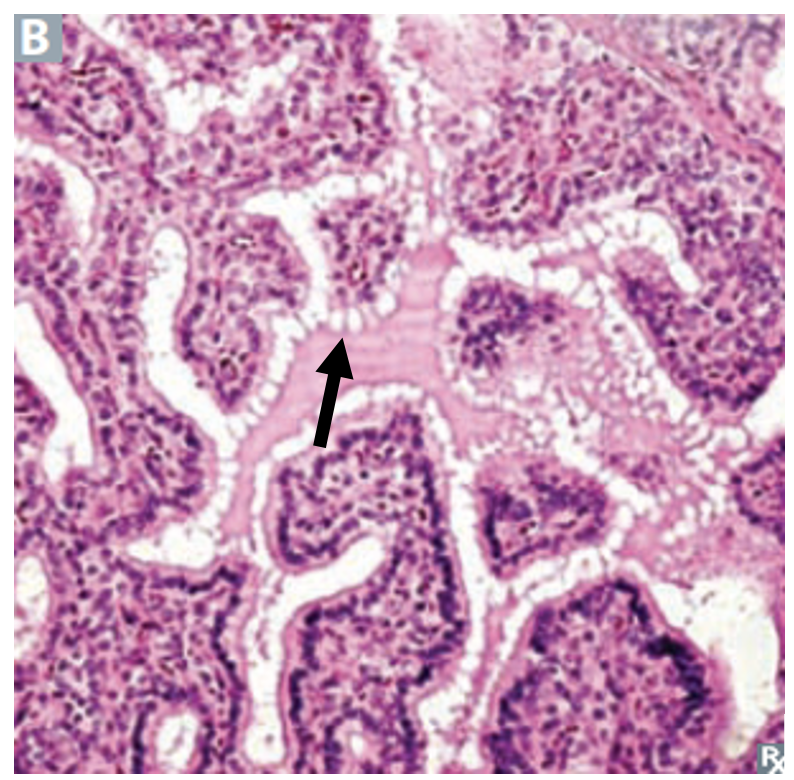
The thyroid consists of spherical follicles, each lined by a layer of cuboidal epithelial cells. Neural crest cells contribute the calcitonin-secreting parafollicular or C cells of the thyroid. (Figures 3a and 3b).
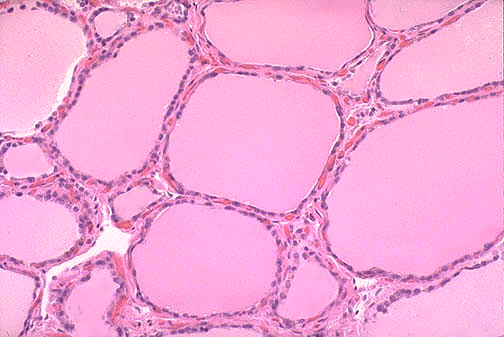
Within the lumen of the follicles is the colloid fluid, an acellular, amorphous gel-like colloid that changes its density depending on the activity of the thyroid gland. The colloid portion of the thyroid contains nascent thyroid molecules attached to a scaffolding protein, called thyroglobulin. The follicles serve as a storage depot for a large supply of thyroid hormone and hormone precursors. These reserves, along with the long half-life of thyroid hormone, ensure steady hormone delivery despite fluctuations in iodine availability or intake, which can limit thyroid hormone production. When inflammatory conditions such as thyroiditis damage thyroid tissue, the release of thyroid hormone stores can cause hyperthyroidism.
The parafollicular C cells are located between the basal lamina and the follicular epithelium and secrete calcitonin in response to a rise in calcium. Calcitonin suppresses resorption of calcium and phosphate from bone. Other cells found in the thyroid include endothelial cells and fibroblasts.
Thyroid hormone synthesis and secretion
Hormone structure
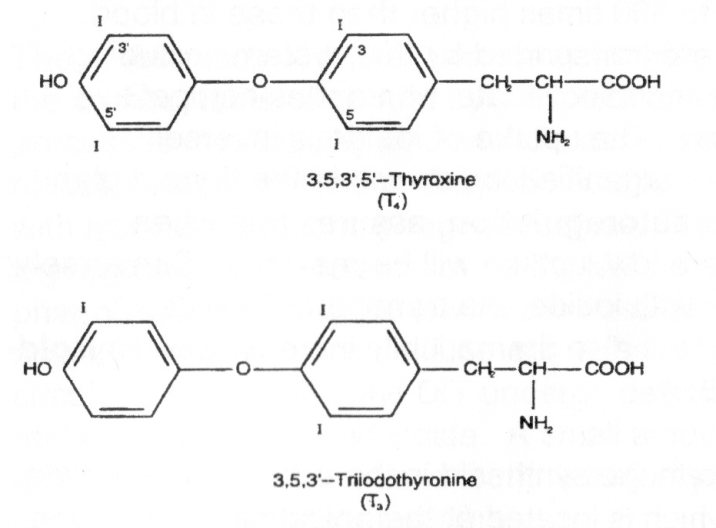
Unlike any other molecules in the body, the two thyroid hormones thyroxine (T4) and triiodothyronine (T3) contain significant amounts of elemental iodine (Figure 4). Sufficient iodine intake is critical for normal thyroid function.
Hormone synthesis
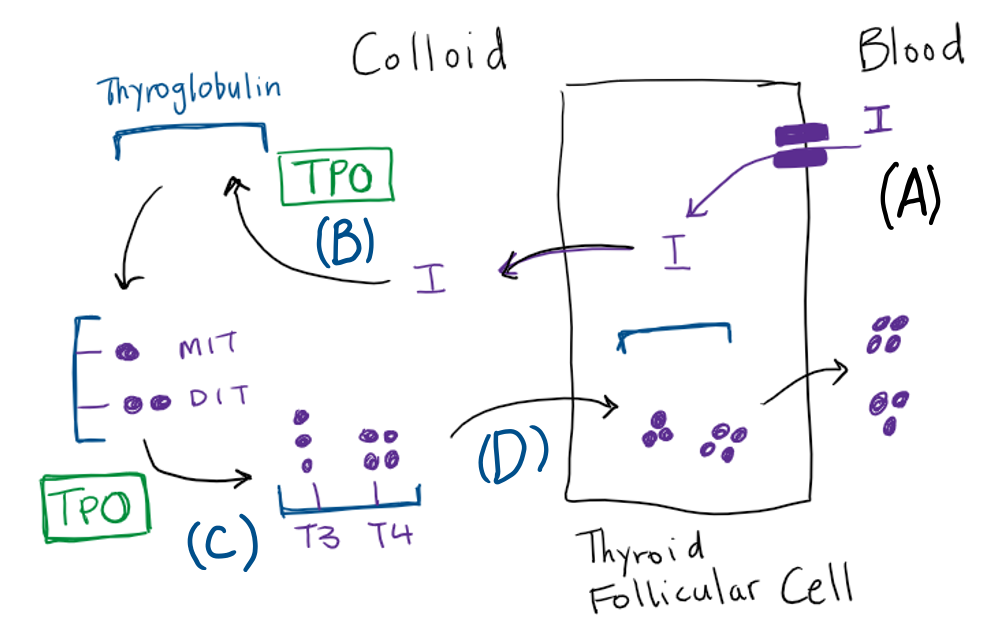
The synthesis and secretion of T4 and T3 consists of the following steps (summarized in figure 5).
Iodide transport
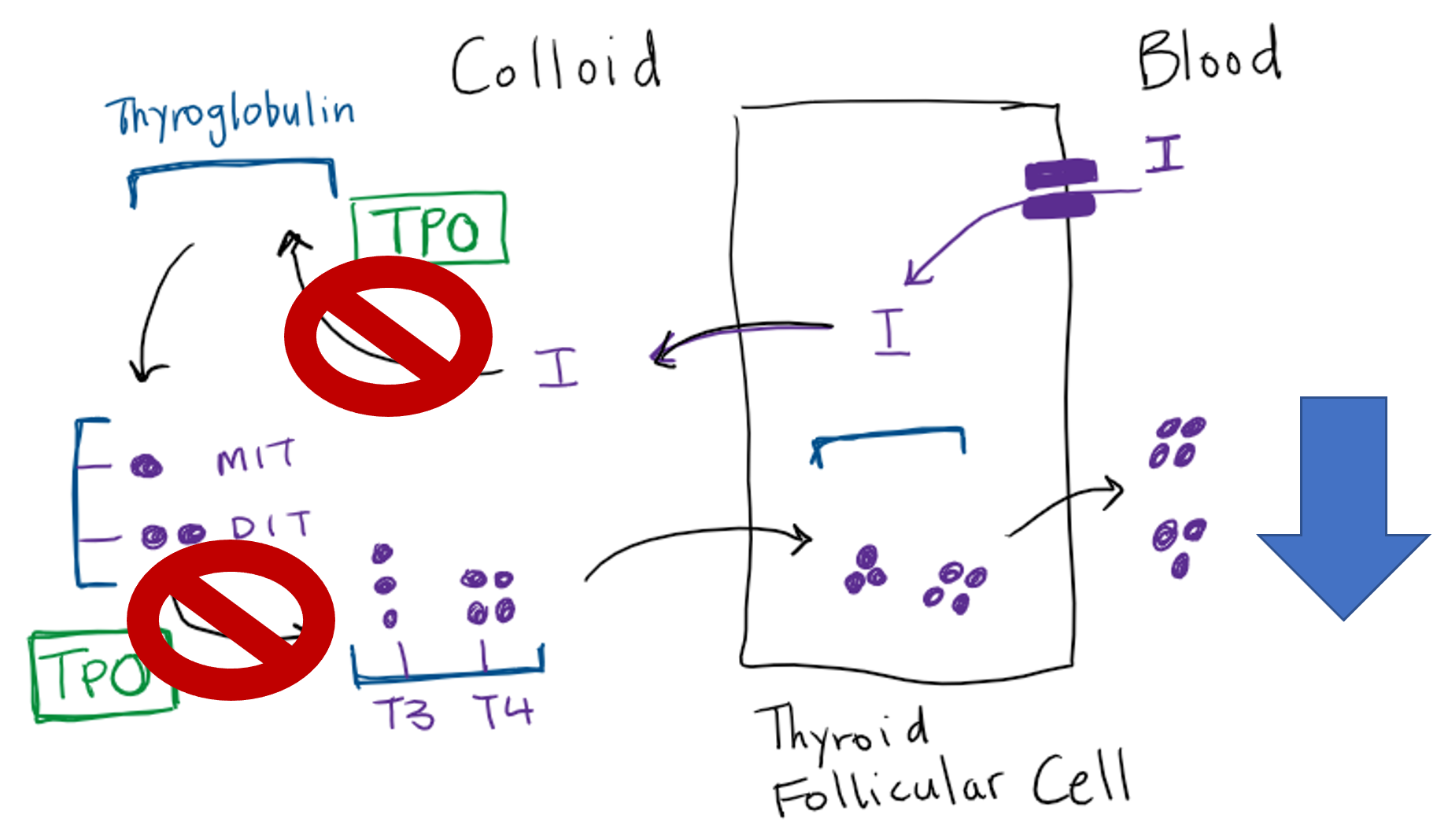
Synthesis of thyroid hormone begins with the transport of iodide across the basolateral plasma membrane of the thyroid follicular cell by the Na+/I– symporter that is driven by a sodium gradient resulting from the action of a Na+/K+ ATPase. Thyroid stimulating hormone (TSH) stimulates iodide transport. The uptake of iodide is inversely proportional to the stores of organified iodide within the thyroid gland. This phenomenon, termed autoregulation, assures that when intrathyroidal iodide stores are low, uptake will be maximal. Conversely, when the thyroid is replete with iodide, the transport of iodide decreases.
Iodination of thyroglobulin
Thyroglobulin is the scaffold upon which thyroid hormone is synthesized. Thyroglobulin is synthesized in the thyroid follicular cells and moved via exocytosis to the colloid space where it is stored. Iodide is moved from the follicular cell to the colloid via the anion transporter protein Pendrin. Thyroid peroxidase (TPO) is the key apical membrane-bound enzyme involved in the synthesis of thyroid hormone. TPO mediates organification, which refers to the oxidation of iodide ions by hydrogen peroxide and their incorporation into tyrosine residues. The tyrosine residues remain part of the thyroglobulin protein at this point. When iodine binds to the carbon in the 3 position within the tyrosine phenol ring, monoiodotyrosine (MIT) is formed, while binding at both the 3 and 5 phenol carbons yields diiodotyrosine (DIT) within thyroglobulin.
Coupling of thyroid hormone precursors
With thyroglobulin as a scaffold, TPO catalyzes the coupling (aka coming together) of a monoiodo- or diiodotyrosine side chain to DIT residues within the thyroglobulin, to form a T3 or T4 residue in thyroglobulin, respectively. These are not true T3 and T4 molecules until released from thyroglobulin via proteolysis).
Mobilization of thyroid hormone from thyroglobulin
Through endocytosis, microvilli on the apical border of the follicular cell engulf and internalize colloid containing iodinated thyroglobulin with the newly formed T4 and T3 residues. These endocytic vesicles fuse with lysosomes to form phagolysosomes. As the phagolysosomes migrate to the base of the cell, proteolysis of thyroglobulin occurs, releasing T4 and T3 from thyroglobulin. Active thyroid hormones diffuse out of the follicular cell into the bloodstream.
Regulation of thyroid hormone
Hormone synthesis
Thyroid hormone synthesis is regulated primarily by TSH (secreted from the anterior pituitary gland) and iodide. TSH stimulates virtually all steps of thyroid hormone synthesis as well as the growth of follicular epithelium. TSH, like most “stimulating hormones”, is released from the pituitary gland as a trophic hormone that causes growth of the end gland with stimulation. Iodide, typically at pharmacologic doses, inhibits the secretion of thyroid hormones, and transiently regulates the uptake of iodide via the sodium/iodide symporter, resulting in reduced organification and synthesis of thyroid hormone, a phenomenon known as the Wolff-Chaikoff effect. This prevents overproduction of thyroid hormone in conditions of high iodine levels. Escape from the Wolff-Chaikoff effect occurs as decreased trapping of iodide restores intrathyroidal iodide stores to normal levels over two to four weeks. If a patient has an underlying condition in which increased iodine uptake occurs (e.g. hyperthyroidism from Graves’ disease or multinodular goiter), escape from the Wolff-Chaikoff effect may worsen the hyperthyroidism. Conversely, patients with underlying thyroid inflammation that may predispose to hypothyroidism, such as with autoimmune thyroiditis, may be unable to effectively “escape” from the Wolff-Chaikoff effect, and may develop permanent hypothyroidism following a large iodine load. Some medications (amiodarone) and IV contrast used for CT scans contain high amounts of iodide.
Pituitary regulation
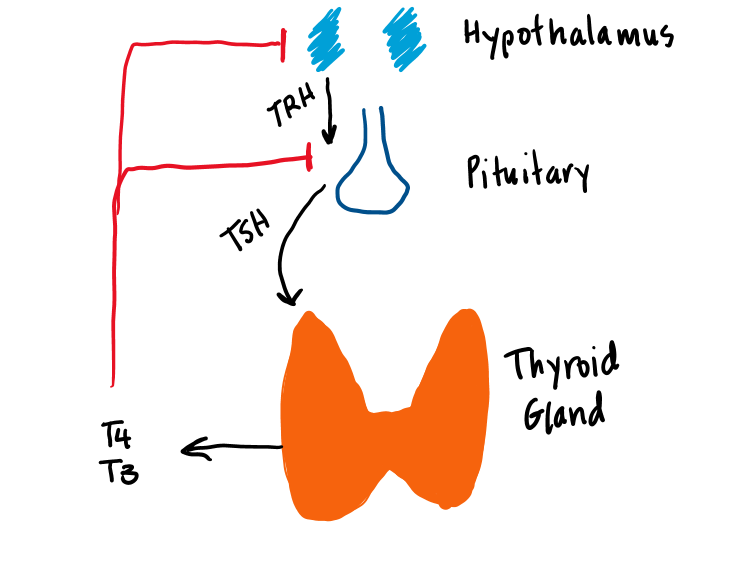
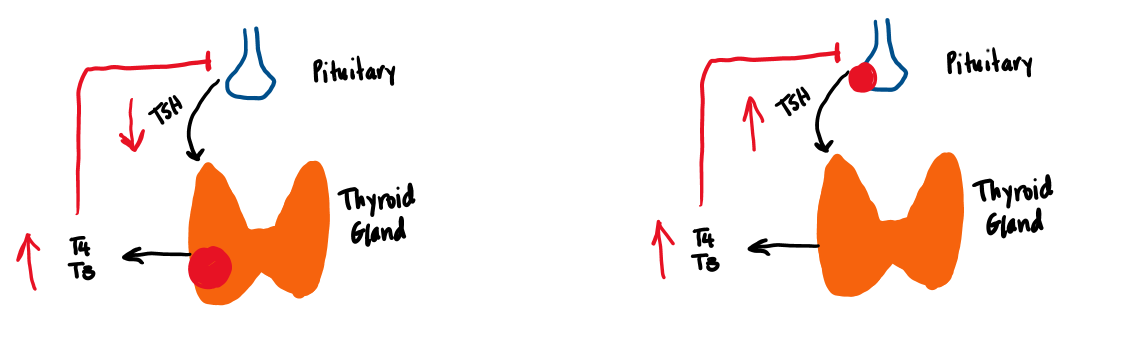
Two basic processes are responsible for maintaining an adequate level of thyroid hormone activity in the body. The first is the regulated secretion of T4 and T3 from the thyroid gland. Thyroid hormone secretion is regulated by a negative feedback loop. The second important regulatory process is the peripheral conversion of T4 to T3.
The first important molecule in the regulation of thyroid hormone secretion is thyrotropin releasing hormone (TRH) (Figure 6). TRH is transported down the pituitary portal system to the thyrotrope cells of the anterior pituitary where it stimulates the secretion of TSH. TSH binds to a G-protein coupled receptor on the thyroid cell, which activates Gs/adenylate cyclase. This produces cyclic AMP, leading to thyroid hormone synthesis and secretion of T4 and T3. T3 feeds back directly on the pituitary to inhibit TSH secretion. T4 indirectly feeds back on the pituitary only after it is converted to T3 by deiodinase.
Peripheral conversion
Only 20% of serum T3 comes directly from the thyroid. The remaining 80% is produced from the peripheral conversion of T4 to T3 by enzymes called deiodinases, largely in the liver and kidney. Because T3 is much more avidly bound by the nuclear thyroid hormone receptor, T4 may be viewed as a prohormone for the active thyroid agent, T3. Although T4-to-T3 conversion is not regulated in the same sense as thyroid hormone secretion, this step is sensitive to caloric balance and the catabolic state of the organism. This topic is discussed in the next section.
Thirty-five percent of circulating T4 undergoes deiodination of its outer ring to form T3 and 45% of T4 undergoes deiodination of its inner ring to form inactive reverse T3 (rT3). Various deiodinase enzymes catalyze these reactions, with affinity for specific iodine molecules, which determines whether the hormone is active T3 or inactive reverse T3 (Table 1):
Type 1 deiodinase (DIO1) is located mainly in the kidneys and liver. Its main role is generating circulating T3, which is available to peripheral tissues, particularly the heart, which does not have any intrinsic deiodinase activity. DIO1 has relatively low affinity for T4.
Type 2 deiodinase (DIO2) is a source of intracellular T3 as well as circulating T3. DIO2 has a much higher affinity for T4 than the DIO1 and can maintain T3 levels even in settings of low T4. DIO2 is found primarily in the pituitary and skeletal muscle. T3 formed by DIO2 in the pituitary is necessary for physiologic feedback regulation of TSH secretion.
Type 3 deiodinase (DIO3) Is important in inactivation of T4 and T3. It is present in the brain and placenta and is enhanced in conditions of thyroid hormone excess and decreased in states of thyroid hormone deficiency.
The balance between T3 and rT3 production determines overall thyroid activity. Most T4 is converted to T3. The remaining 20% of circulating T4 is mostly metabolized in the liver and secreted in the bile with reclamation of liberated iodide from the gastrointestinal tract.
|
Deiodinase |
Location |
Action |
Function |
|
Type 1 |
Liver, kidneys |
Activating, T4 > T3 |
Source of circulating T3 |
|
Type 2 |
Brain, pituitary, thyroid, fat, muscle, placenta |
Activating, T4 > T3 |
Tissue production of T3, feedback regulation TSH in pituitary |
|
Type 3 |
Brain, placenta skin |
Inactivating, T4 > rT3 |
Inactivation T4 and T3 in states of excess thyroxine |
Thyroid hormone action
Cellular signaling
Free thyroid hormone (both T3 and T4) passively diffuses through the cell membrane across the cytoplasm to a receptor in the cell nucleus. T4 is converted to T3 within the cell by either DIO1 or DIO2. The T3 receptor belongs to the “nuclear receptor” superfamily of steroid hormone receptors that also includes glucocorticoids, estrogen, aldosterone, vitamin D, and retinoid acid receptors.
Thyroid hormone receptors affect gene expression by binding to DNA sites called thyroid response elements (TREs), which are located upstream to promoters involved in gene transcription (Figure 7). In the absence of T3, the T3 receptor binds to corepressors and TREs, suppressing thyroid-hormone-regulated gene transcription. When T3 binds to the ligand-binding domain of the T3 receptor, it releases the corepressors, and binds coactivators. The newly formed heterodimeric receptor-RXR-TRE complex stimulates the transcription of mRNAs, which are eventually translated into proteins that mediate the effects of thyroid hormone.

Metabolic effects
Thyroid hormone plays a role in growth and maturation in multiple species. In humans, thyroid hormone is essential for the development of the central nervous system of the fetus and the neuro-intellectual growth of the neonate and child. Absence of thyroid hormone in utero and during the neonatal period leads to congenital defects including cognitive impairment, deafness, mutism, and dwarfism. Thyroid hormone is necessary for normal linear growth and bone maturation and also allows for the secretion of growth hormone.
In adult animals, thyroid hormones exert a multitude of effects, which collectively ensure metabolic stability. They help maintain body temperature, metabolic cycles, normal glucose and lipid metabolism, as well as normal catecholamine signaling. Thyroid hormone increases cholesterol degradation due to an increase in hepatic low-density lipoprotein (LDL) receptors. This enhances the response to catecholamines and increases metabolic rate. Thyroid hormones increase heart rate and contractility. They stimulate bone remodeling and gastric motility.
Thyroid function tests
The presence of abnormal thyroid function in an otherwise healthy population is relatively common but varies significantly with age and other demographic characteristics. The Colorado Thyroid Disease Prevalence Study, a large, population-based epidemiologic survey of healthy adults, estimated the prevalence of elevated TSH to be 9.5% and low TSH to be 2.2%. Higher TSH levels are associated with female sex, advancing age, and presence of thyroid antibodies (particularly anti-TPO antibody).
TSH levels
TSH is the most useful screening test of thyroid function because it reflects the body’s own regulation of thyroid hormone status. Subtle changes in thyroid status lead to reciprocal changes in TSH due to negative feedback. If the TSH is outside the normal range, then free (unbound) thyroxine (T4) should be measured. Only free circulating thyroid hormone is biologically active because, unlike hormone bound to protein, it can cross the cell membrane and bind to receptors in the cell nucleus. Total thyroid hormone levels (i.e. bound plus free) can be affected by the alterations in TBG (thyroid binding globulin), a carrier protein that binds to thyroid hormone in the blood. This topic will be covered in more detail in the section on thyroid function and pregnancy.
Elevated TSH with a low free T4 indicates primary hypothyroidism. A TSH below the normal range with high free T4 is found in primary hyperthyroidism. There are two situations in which TSH is not a reliable measure of thyroid function – If pituitary or hypothalamic disease is suspected, then free T4 and TSH should be checked. A low free T4 and an inappropriately low or normal TSH may be suggestive of pituitary dysfunction. The second situation where TSH is not reliable is in cases of rapidly changing thyroid hormone levels, such as following thyroid surgery. TSH can take 6-8 weeks to reach a new steady state following a change in thyroid hormone levels, so changes in TSH will always lag. In situations where thyroid hormone levels are in flux, it is best to monitor free T4 levels. The expected results of TSH testing in different conditions of abnormal thyroid hormone are shown in Table 2.
|
Condition |
Location of abnormality |
Thyroid hormone level (free T4 or T3) |
TSH level |
|
Hyperthyroidism |
Thyroid (1°) |
High |
Low |
|
|
Pituitary (2°) |
High |
High |
|
Hypothyroidism |
Thyroid (1°) |
Low |
High |
|
|
Pituitary (2°) |
Low |
Low |
Thyroid hormone levels
Both T4 and T3 normally circulate tightly bound to serum binding proteins. Only about 0.05% of circulating T4 and 0.5% of circulating T3 exist in a free, unbound, metabolically active form in the serum. The extensive binding of T4 and T3 maintains a hormone reservoir and prevents excretion of thyroid hormone by the kidney. Eighty percent of T4 is bound to thyroxine-binding globulin (TBG). The remaining 20% of T4 is bound to albumin and transthyretin (prealbumin). The binding affinity of TBG for T4 is ten times that for T3. Therefore, the proportion of T3 that circulates in the free state is higher than that of T4. One can measure both the protein bound serum levels of thyroid hormone, known as the total T4 and T3 levels, as well as the free hormones levels, denoted free T4 and free T3. The plasma TBG concentration, and thus the total level of thyroid hormone in the blood, is strongly influenced by the functional status of the liver, the nutritional status of the individual, and the presence of estrogen and other drugs (Table 3). In the presence of elevated TBG, total thyroid hormone levels will be elevated. Similarly, low total thyroid hormone is seen with low TBG levels, but in both cases, free thyroid hormone levels remain normal.
| Causes of increased TBG and high total T4 |
| Estrogens (e.g. pregnancy, birth control pills |
| Congenital X-linked disorder causing elevated TBG levels |
| Causes of decreased TBG and low total T4 |
| Glucocorticoids |
| Protein loss: malnutrition, wasting, liver failure, nephrotic syndrome |
Thyroid pathology
Hypothyroidism
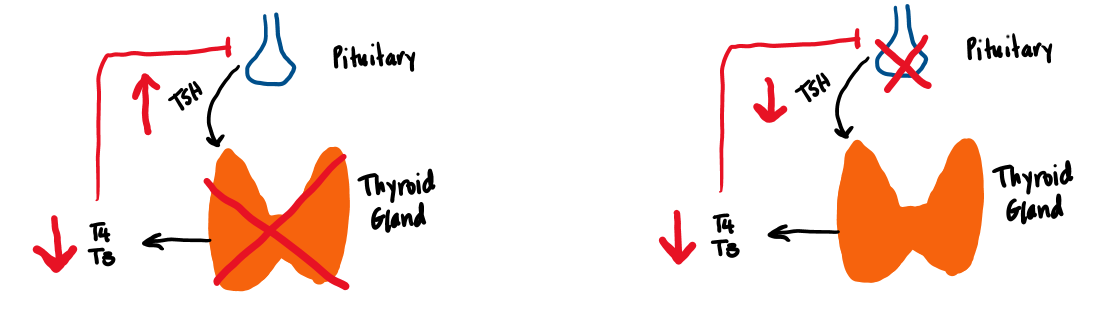
Hypothyroidism is a state of thyroid hormone deficiency, which results in a slowing of metabolic processes. Primary hypothyroidism refers to thyroid gland failure and is characterized by a low free T4 and a high TSH (due to reduced negative feedback of T4 and T3 on the pituitary). Secondary hypothyroidism is characterized by insufficient TSH production by the pituitary, while tertiary hypothyroidism occurs when the hypothalamus does not secrete enough TRH. Primary hypothyroidism accounts for about 99% of cases of hypothyroidism, while central hypothyroidism (secondary and tertiary) account for less than 1%. (Figure 8).
Etiology of Hypothyroidism
The most common cause of primary hypothyroidism worldwide is iodine deficiency due to inadequate daily dietary intake of iodine. Iodine deficiency is particularly harmful in pregnancy, as the developing fetus is dependent on maternal iodine for thyroid hormone production. Failure to adequately treat iodine deficiency during pregnancy can result in severe intellectual disability for the infant, deaf mutism, and gait disturbances. Affected infants may also have short stature and congenital hypothyroidism. Iodine deficiency is considered a global public health problem, and salt iodization programs have been implemented worldwide to combat this problem. Table 4 lists causes of hypothyroidism.
Congenital hypothyroidism occurs in 1:2000 to 1:4000 newborns and is the most common preventable cause of intellectual disability worldwide. Most cases are sporadic, due to thyroid dysgenesis, although there are some inherited causes of congenital hypothyroidism (beyond the scope of this course). Most newborns have no symptoms of hypothyroidism at birth, since maternal T4 can cross the placenta, which provides newborns about 25-50% of normal T4 levels at time of birth. If congenital hypothyroidism is not detected at time of birth, through newborn screening, the children will start to manifest symptoms of hypothyroidism over the first few months of life, with lethargy, feeding problems, hypotonia and prolonged jaundice. Inadequate treatment of congenital hypothyroidism results in decreased cognitive outcomes compared to their peers.
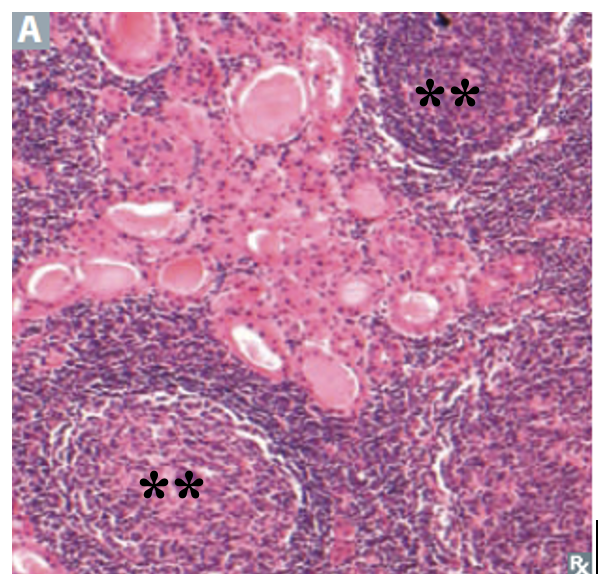
In the United States, where dietary iodine supplementation and neonatal thyroid screening have been implemented, the most common cause of acquired hypothyroidism is chronic lymphocytic (Hashimoto’s) thyroiditis. Chronic lymphocytic (Hashimoto’s) thyroiditis is an autoimmune process where autoantibodies are directed against the TPO enzyme essential to thyroid hormone synthesis. Some individuals may have antibodies against thyroglobulin as well. Thyroid destruction results from both cellular and humoral mechanisms, resulting in a cytopathology characterized by lymphocytic infiltrates with germinal centers and fibrosis (Figure 9). Another finding is the Hurthle cell, which is a large eosinophilic cell with an eccentric nucleus and a high mitochondrial content. Although there are identified factors that suggest genetic susceptibility (these patients often have a positive family history of autoimmune disease), there are no well-defined environmental risk factors.
Other acquired causes of hypothyroidism are radioiodine ablation or surgical treatment for multinodular goiter or thyroid cancer. Certain drugs (such as amiodarone or IV contrast) may also cause hypothyroidism (from failure to escapte the Wolff-Chaikoff effect). There are both synthetic and naturally occurring blockers of several steps in thyroid hormone synthesis that can result in hypothyroidism as well. Thiocyanates, found in cabbage, turnips, and rutabaga can competitively inhibit iodide transport and, if ingested raw in sufficient quantities, can lead to goiter and hypothyroidism. Lithium (a medication used for mood disorders) also interferes with various steps in thyroid hormone synthesis and release. Iodine, as discussed in the section on thyroid hormone synthesis, can also temporarily block thyroid hormone production (Wolff-Chaikoff effect).
| Causes of Primary Hypothyroidism |
| Chronic lymphocytic thyroiditis (Hashimoto’s) |
| Iodine deficiency |
| Iatrogenic (thyroidectomy or radioiodine ablation) |
| Drugs (amiodarone, lithium, thionamides, IV contrast) |
| Infiltrative diseases (sarcoidosis, hemochromatosis) |
| Causes of Central (secondary and tertiary) hypothyroidism |
| Pituitary or CNS mass lesions (and their surgical excision) |
| Radiation |
| Trauma |
| Infiltrative diseases (sarcoidosis, IgG4RD, hemochromatosis) |
| Pituitary apoplexy |
| Autoimmune lymphocytic hypophysitis |
| Pituitary infarction (Sheehan’s syndrome) |
Central hypothyroidism is a condition of either lack of TSH production from the pituitary (secondary hypothyroidism) or lack of TRH secretion from the hypothalamus (tertiary hypothyroidism) to stimulate the thyroid gland to produce thyroid hormone. The T4 is low, and TSH is inappropriately low or normal in cases of central hypothyroidism. Central hypothyroidism is caused by hypothalamic and pituitary tumors or their surgical excision, radiation therapy, trauma, infiltrative disease (i.e. hemochromatosis or sarcoidosis, as well as some fungal infections), pituitary apoplexy (infarction), or autoimmune destruction of the anterior pituitary (autoimmune lymphocytic hypophysitis). It is often accompanied by other pituitary hormonal disorders.
Clinical Symptoms of Hypothyroidism
Patients with hypothyroidism commonly report symptoms of fatigue, weight gain, and cold intolerance due to decreases in basal metabolic rate and thermogenesis. Other symptoms include muscle cramps, paresthesia, depression, dry skin, and hair loss. Physical findings include bradycardia, hair loss, coarse scalp hair, dry skin, macroglossia (tongue enlargement), voice hoarseness, and occasionally goiter. The accumulation of glycosaminoglycans and fluid in the dermis and interstitial tissues leads to puffy, sallow facies and peripheral edema.
In children, thyroid hormone deficiency typically presents as declining growth velocity, and may be present years before other symptoms occur. Skeletal maturation is delayed, as is pubertal development. Students may also have a decline in school performance. The most common exam finding is an enlarged thyroid (called a goiter).
Myxedema coma is the most severe form of untreated hypothyroidism characterized by hypothermia, delirium, seizures, coma, bradycardia, respiratory failure, and total body fluid overload (i.e. periorbital edema, ascites, pleural effusions, and/or pericardial effusions). There is often a precipitating event, such as infection, congestive heart failure, myocardial infarction, hypothermia, trauma or surgery. Supportive therapy includes treating hypothermia, respiratory failure, and hypotension. The hormone deficiency itself is treated initially with high dose parenteral T4 (see next section), which is later reduced to replacement doses administered orally.
Treatment of Hypothyroidism
Hypothyroidism is treated with hormone replacement with synthetic T4, which is converted to T3 in the periphery. The name of this medication is levothyroxine. T4 is preferable to T3 for treatment of hypothyroidism because of its longer half-life (7 days for T4 versus one day for T3) and thus, steadier hormone levels. In young, healthy patients, it can be initiated at approximately 1.6-1.7 mcg/kg/day. In the frail elderly or those with underlying cardiovascular disease, the estimated full dosage is lower (1.3 mcg/kg/day), and the starting dose is low, i.e. 25-50 mcg/day. For children, the dose is considerably higher (2 – 6 mcg/kg/day based on age, with younger children requiring higher doses). A TSH level should be repeated six to eight weeks after a change of dosage, and the goal TSH level should be within the normal range. Medications or supplements that interfere with the absorption of thyroid hormone include iron, calcium carbonate, cholestyramine, and aluminum-containing antacids. Patients should be advised to separate dosing of these interfering medications from levothyroxine by at least four hours. Dosage requirements increase during pregnancy, and pregnant women with underlying hypothyroidism often need increased levothyroxine therapy and frequent blood thyroid tests for monitoring.
Hyperthyroidism
Hyperthyroidism is a state of thyroid hormone excess, which results in a hypermetabolic state. Common causes include primary hyperthyroidism (in which the thyroid itself is producing too much thyroid hormone), various forms of thyroiditis (thyroid inflammation), and ingestion of excess thyroid hormone. Struma ovarii (production of thyroid hormone by an ovarian teratoma that contains thyroid tissue) is an extremely rare cause of thyroid hormone excess. All of these causes are characterized by an elevated T4 and a low TSH level (figure 10). Another rare cause of hyperthyroidism is a TSH-producing pituitary adenoma (central hyperthyroidism, aka TSH-oma). This is characterized by elevated TSH with elevated free T4. Because of the similarity between TSH and a hormone that increases in pregnancy (human chorionic gonadotropin, hCG), a temporary gestational hyperthyroidism may develop with increasing hCG levels during pregnancy causing a thyrotoxicosis (discussed below). There are no known hypothalamic causes of hyperthyroidism.
Etiology of Hyperthyroidism
Primary hyperthyroidism is caused by Graves’ disease, toxic multinodular goiter, and toxic nodules.
Graves’ disease is the most frequent cause of hyperthyroidism and is more common among younger women. It is an autoimmune disorder caused by an antibody directed against the TSH receptor (TSH-receptor antibody (TRAb), or thyroid-stimulating immunoglobulin (TSI) resulting in stimulation of thyroid cells to produce excess hormone. The overstimulation of the thyroid in Graves’ disease typically results in a large, highly vascular, diffuse goiter. Microscopically, there are numerous small follicles lined by extremely hyperplastic epithelia interspersed with lymphocytes. The follicular cells appear tall and crowded. Colloid has scalloped margins (figure 11).
Toxic multinodular goiters and solitary toxic adenomas result from diffuse or focal hyperplasia of thyroid follicular cells that function independently of TSH regulation. Activating mutations of the genes for the TSH receptor have been identified as a cause in some cases. Toxic multinodular goiter is more common with increasing age and in areas where iodine intake is relatively low.
| Primary hyperthyroidism |
| Graves’ disease |
| Toxic multinodular goiter |
| Toxic single nodule/adenoma |
| Thyroiditis |
| Secondary hyperthyroidism |
| TSH-secreting adenoma |
| Other causes of hyperthyroidism |
| Ingestion of thyroid hormone |
| Excess iodine |
| Struma ovarii |
| Gestational thyrotoxicosis |
Diagnosis of Hyperthyroidism
The key diagnostic test to distinguish the various causes of hyperthyroidism is the radioiodine uptake and scan, which assesses iodine turnover by the thyroid gland. A small dose of radioactive iodine (either I-131 or I-123) is administered orally, and the uptake of the dose is measured at 4 and 24 hours. The scan provides an image of the radioiodine uptake amount and pattern in the thyroid gland. Diffuse increased uptake is consistent with Graves’ disease, reflecting increased activity of the entire gland due to stimulation of the TSH receptor by autoantibodies. The concentration of iodine in one or more localized “hot” regions (with suppression of surrounding thyroid activity) may be suggestive of toxic nodule(s). The damaged inflamed thyroid follicles in thyroiditis do not have significant radioiodine uptake because no excess hormone is being synthesized as hyperthyroidism from thyroiditis comes from release of preformed hormone. There also may be low radioiodine uptake in exogenous thyroid hormone administration and iodine-induced hyperthyroidism. Figure 12 shows the different patterns of radioactive iodine uptake for different causes of hyperthyroidism.
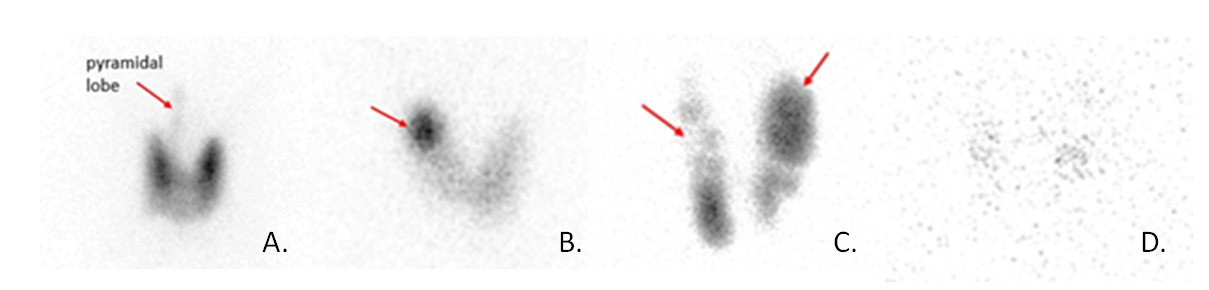
In some situations, obtaining a radioiodine uptake and scan may not be possible, such as in a pregnant or nursing woman, or if a patient has recently had a large iodine load such as with IV contrast. In such cases, measurement of the antibodies that cause Graves’ disease can be helpful. The antibodies measured are thyrotropin receptor antibody (TRAb) and thyroid stimulating immunoglobulin (TSI). The antibody titers are positive in over 90% of patients with Graves’ disease and concurrent symptoms of thyrotoxicosis. Sensitivity and specificity of available assays are both over 90-95%.
Clinical Manifestations of Hyperthyroidism
Hyperthyroid patients complain of weight loss, heat intolerance, and palpitations. On physical exam, the patient may appear to stare due to increased tone of sympathetically-innervated ocular muscles. They may also have tachycardia, a fine tremor, and hyperreflexia. In addition, there are physical exam findings related to focal inflammation that are specific to Graves’ disease. Graves’ ophthalmopathy (caused by a lymphocytic infiltration of extraocular tissues) may include protrusion of the globe and impaired extraocular muscle movement. Graves’ dermopathy (also called pretibial myxedema) refers to thickening of the skin overlying the anterior tibial or ankle regions due to accumulation of glycosaminoglycans. The thyroid gland in Graves’ disease is enlarged and homogenous. Patients with toxic multinodular goiter may have a “lumpy bumpy” goiter that can be palpable. Subacute thyroiditis may cause local thyroid tenderness and may be accompanied by preceding symptoms of upper respiratory tract infection. A painless form of thyroiditis with transient hyperthyroidism is particularly common in patients after childbirth (postpartum thyroiditis).
Hyperthyroidism occurs in 1:5000 children, with most cases due to Graves’ disease. Children with hyperthyroidism often present with failure to gain weight or weight loss, despite increased appetite. Mood swings and behavior problems are more common in children than adults. They have shorter attention span, poor sleep and decline in school performance. On exam, most will have a diffuse goiter.
Thyroid storm is a form of severe, decompensated, hyperthyroidism characterized by fever, tachycardia, congestive heart failure, gastrointestinal symptoms, and altered mental status. There is usually a precipitating factor, such as surgery, trauma, cerebrovascular accident, myocardial infarction, diabetic ketoacidosis, infection, or discontinuation of antithyroid medications. Treatment of thyroid storm includes: (1) therapy to control thyroid hormone synthesis (thionamides, discussed below); (2) therapy to block the peripheral conversion of T4 to T3 (PTU, beta blockers, corticosteroids); and (3) supportive measures (treating the precipitating cause, antipyretics, cooling blankets, intravenous fluids).
Treatment of Hyperthyroidism
Symptoms of hyperthyroidism will improve with nonspecific treatment of the hyperadrenergic state using beta blockers (this medication class will be covered later in the Foundations phase). However, beta blockers will not correct the underlying cause. Other treatments are needed to treat the thyroid hormone excess. Graves’ disease and autonomous nodules are both conditions of thyroid hormone overproduction and can be treated with long-term administration of thionamide drugs, such as methimazole (MMI) and propylthiouracil (PTU) that block thyroid hormone synthesis (Figure 13). The thionamides inhibit both the organification of iodine and the coupling of DIT and MIT to form thyroid hormone. The most common side effect of these medications is rash. Agranulocytosis (a serious condition that occurs when there is an extremely low number of granulocytes in the blood). Granulocytes are an important part of the immune system and help the body fight infection. Agranulocytosis is seen in <1% of patients. Methimazole can cause a reversible cholestatic liver injury, while PTU can cause fulminant hepatic necrosis. While these are both rare, hepatic injury is more common with PTU and therefore it is not recommended for treatment in hyperthyroidism except during the first trimester of pregnancy. Methimazole is also associated with more severe teratogenic effects, so it is generally avoided during first trimester of pregnancy. For women with hyperthyroidism who need treatment during pregnancy, they should be treated with PTU during the 1st trimester, and then switch to methimazole during 2nd trimester, once organogenesis is complete. Thionamides are the treatment of choice for children with Graves’ disease, in the hope that they will achieve remission and avoid lifelong hypothyroidism (which occurs after radioactive iodine ablation, see below).
Radioactive iodine (RAI) is a definitive/permanent treatment for Graves’ disease and autonomous nodules. High dose radioactive iodine is given, which is taken up by the hyperfunctioning areas of the thyroid. The radiation damage destroys the thyroid cells and renders them unable to produce thyroid hormone. Occasionally treatment of Graves’ disease or autonomous nodules requires surgery, either because patients are unable or unwilling to take medication or undergo radiation, or due to compressive symptoms from a large goiter. Either RAI or surgery are acceptable alternatives to thionamides for children who are unable to tolerate the medications, or do not achieve remission.
Thyroiditis does not require specific treatment beyond symptomatic therapy. Once the thyroid hormone stores are depleted, thyroid hormone levels gradually decline. The follicular cells heal, and most people will return to normal thyroid function. Occasionally the damage to the thyroid is so severe, that patients become hypothyroid. Thus, it is important to monitor thyroid hormone function following a bout of thyroiditis.
The Non-Thyroidal Illness Syndrome (NTIS)
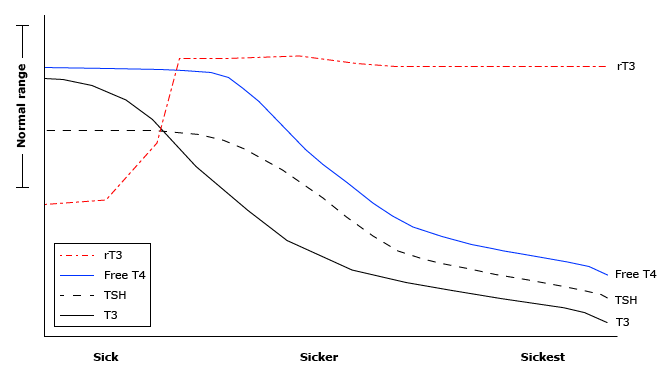
Not all thyroid hormone abnormalities are due to underlying thyroid disease. Thyroid hormone levels are often abnormal during episodes of illness or starvation. Starvation or illness leads to rapid inhibition of DIO1 activity in the periphery. This results in decreased activation of T4, decreased circulating levels of T3 and increased reverse T3 levels. High levels of DIO2 in the pituitary and brain continue to convert T4 into T3. This ensures adequate T3 levels in the brain, even in states of malnutrition and illnesses, which maintains normal TSH levels in mild illness.
During prolonged starvation or illness, T4 levels may also drop. This is initially seen as low total T4 as there is decreased protein synthesis by the liver, resulting in a decrease in thyroid hormone binding proteins, but maintenance of normal free T4. With severe illness however, the hypothalamus decreases TRH release. This leads to a decrease in TSH release, and low T4 and T3 levels (figure 14).
Some people speculate that this is an adaptive response to conserve energy in times of stress by down-regulating metabolic processes. Treatment of the thyroid hormone abnormalities is not necessary and in fact may be harmful. With non-thyroidal illness, thyroid function tests normalize 6-8 weeks after resolution of the underlying illness. TSH levels may rebound transiently above the normal range during recovery after non-thyroidal illness. It is important to recognize this in hospitalized patients to avoid inappropriate treatment of thyroid lab abnormalities.
Thyroid disease and pregnancy
Thyroid hormone physiology changes dramatically in pregnancy and affects people who are pregnant regardless of prior diagnosis of thyroid dysfunction. Two main changes contribute to changing thyroid hormone physiology in pregnancy: rising human chorionic gonadotropin (hCG) levels and change in thyroid binding globulin.
Human chorionic gonadotropin is very similar to TSH. When hCG levels are high (during the first trimester), there is binding of hCG at the TSH receptor with downstream effects leading to increased thyroid hormone synthesis. This increased thyroid hormone exerts negative feedback at the hypothalamus and pituitary glands and leads to decreased TRH and TSH secretion. Therefore, a normal pattern of thyroid labs in early pregnancy will show mildly elevated (or high-normal) thyroid hormone levels and low (or low-normal) TSH. hCG levels peak in the first trimester and decline through the rest of pregnancy. With this decline TSH levels normalize. Rarely (in 1-3% of pregnancies), gestational hyperthyroidism will develop. Gestational hyperthyroidism occurs when the levels of hCG are high enough to cause thyrotoxicosis. It occurs in pregnancies with very high hCG levels such as hyperemesis gravidarum and multiple gestation.
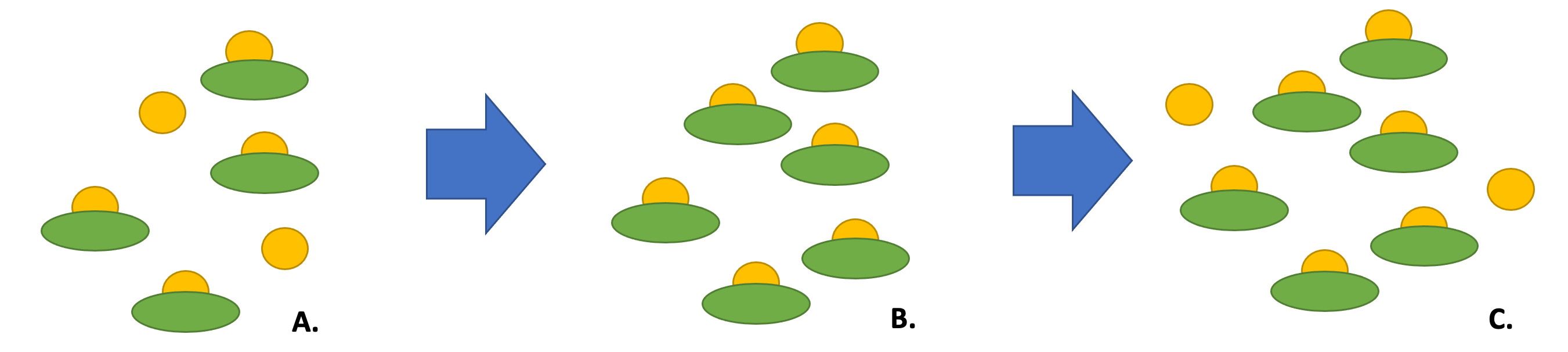
As described in a previous section, the rise in estrogen levels during pregnancy leads to increased thyroid binding globulin. An increase in thyroid binding globulin leads to transient decrease in free hormone levels (because more of the thyroid hormone is bound). In a person with a normally functioning thyroid gland, the hypothalamus and pituitary compensate for this transient (and subclinical) change by a rise in TSH that leads to increased thyroid hormone synthesis. Therefore, the free levels remain equivalent to the non-pregnant state, but the total hormone levels have increased (Fig 15).
In people with pre-existing hypothyroidism who become pregnant, the thyroid gland is unable to compensate for the decrease in free hormone levels due to the increase in TBG. Therefore, people with hypothyroidism on levothyroxine generally need to increase the dose by 1.5x during pregnancy to compensate for increase in binding globulins.
Thyroid nodules
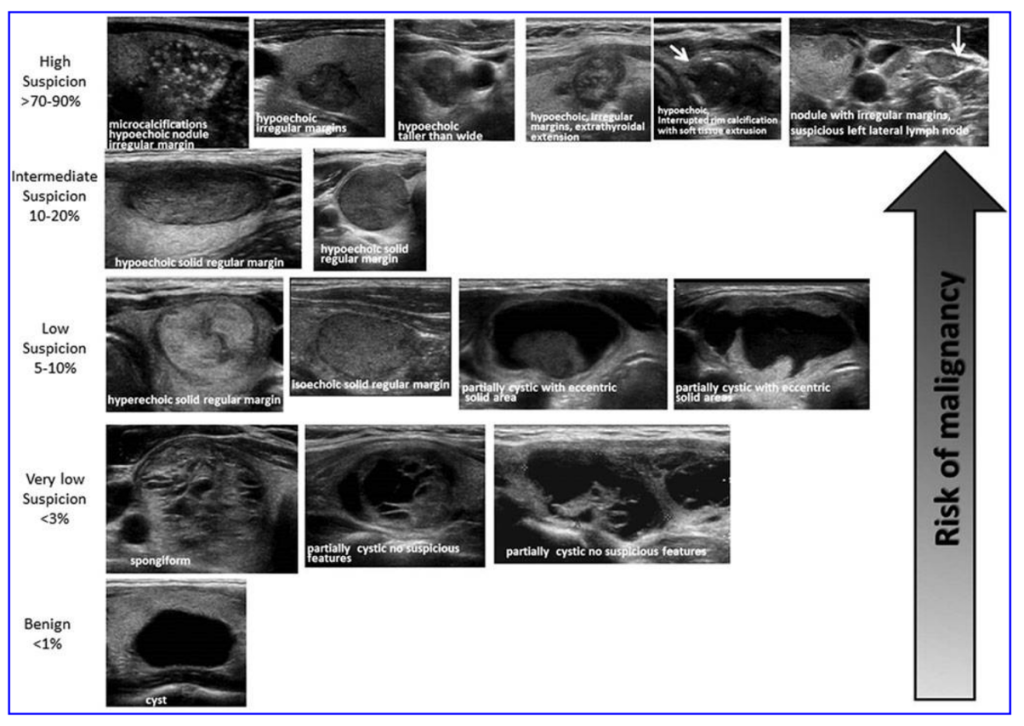
Thyroid nodules greater than 1 cm in size may be palpated on physical exam or, more commonly, detected incidentally on an imaging test done for other reasons, such as a CT scan or ultrasound. The causes of thyroid nodules are multinodular goiter (toxic or nontoxic), benign follicular adenoma, cysts, and malignancy. Most thyroid nodules are benign, only 5-6% of nodules are malignant. Risk factors and symptoms for malignancy include age (nodules are more likely to be malignant in young patients), family history of medullary thyroid cancer, history of head and neck irradiation, a fixed (non-mobile) nodule, obstructive symptoms (dysphagia, hoarseness), and cervical lymphadenopathy. The patient with a thyroid nodule should have a TSH level measured. If the TSH is low, a radioiodine uptake and scan should be ordered to evaluate for hyperfunctioning nodules. If a hyperfunctioning (aka “hot” or toxic) nodule is found on the scan, then radioiodine treatment or surgical excision should be considered to treat the hyperthyroidism, as it is extremely rare for autonomous nodules to be cancerous. If the TSH is normal or elevated, then a thyroid ultrasound should be ordered, and the patient should be referred to an endocrinologist, who will consider biopsy of the nodule by fine needle aspiration (FNA) biopsy on the basis of size (> 1-1.5 cm) and ultrasound characteristics (Figure 16), as well as other risk factors. If the cytopathology from the FNA suggests malignancy, then the patient is referred for surgery. In some cases, the cytopathology is neither benign nor clearly suggestive of malignancy. Gene microarray testing is now available that can help guide the decision to refer a patient to surgery (if the gene array suggests higher risk) or to watchful monitoring (lower risk gene array).
Thyroid Cancers
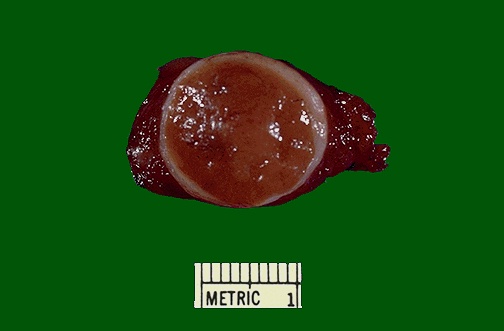
Differentiated thyroid cancer (DTC) is a type of thyroid cancer in which the cancer cells look similar to normal thyroid cells when viewed under a microscope. Most differentiated thyroid cancers tend to grow slowly, can be treated, and can usually be cured. The differentiated thyroid malignancies include papillary and follicular carcinomas. Histologically, the papillary subtype is distinguished by a frond-like histology, the presence of small concentrically layered calcifications called psammoma bodies, and large, pale nuclei with intranuclear inclusion bodies. Papillary carcinoma is often multicentric within the thyroid gland and tends to metastasize to local lymph nodes. It may become more aggressive in older patients, spreading to the muscles and the trachea, and even in some cases the lungs. Follicular carcinoma appears microscopically as abundant small follicles lined by cuboidal cells with large nuclei, with possible capsular or vascular invasion. The distinction between a benign follicular adenoma (Fig 17) and malignant follicular carcinoma is made based on the absence (benign) or presence (malignant) of capsular invasion. This distinction can only be made on surgical pathology, so patients with suspected follicular lesions often require surgery for a final diagnosis. Follicular carcinoma metastasizes hematogenously to distant sites, including lung and bone. Figure 18 shows histology of the most common thyroid malignancies.
Two staging systems are used for differentiated thyroid cancer (papillary thyroid carcinoma and follicular thyroid carcinoma). These include the TNM staging system (tumor-node-mets) to predict disease specific mortality and the ATA (American Thyroid Association) staging system to predict risk for recurrence. Because disease-specific mortality from differentiated thyroid cancer is very low. The ATA guidelines are much practical and group DTC into low, intermediate, and high risk for recurrence.

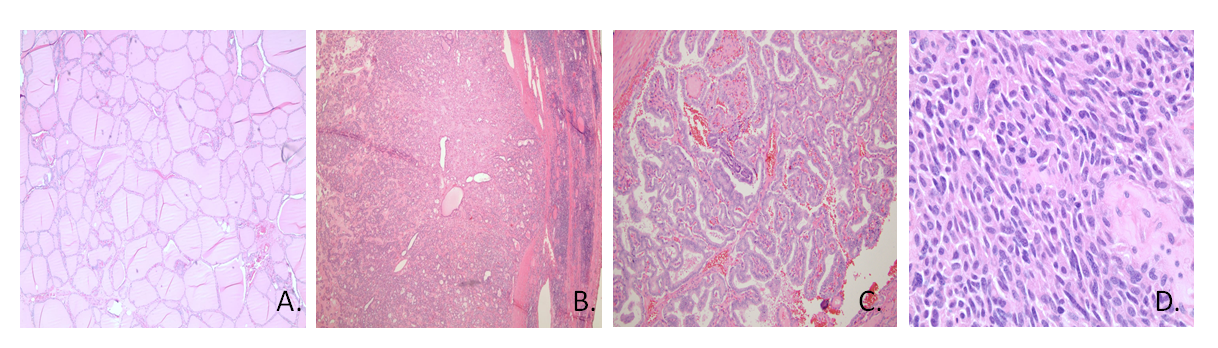
Medullary thyroid carcinoma is a tumor of the parafollicular or C-cells that produce calcitonin. On histology, cells are spindle shaped with amyloid present. The tumor may occur sporadically or in a familial pattern as part of the type 2 multiple endocrine neoplasia (MEN) syndrome, in which case it is caused by a mutation of the RET proto-oncogene. This mutation can be screened for in family members and when present prophylactic thyroidectomies can be performed in affected individuals. The production of calcitonin by the malignant cell type provides a biochemical marker for diagnosis and assessment of tumor response to therapy, but despite often high levels of calcitonin, these patients do not typically have abnormal calcium levels. Treatment of medullary thyroid carcinoma includes total thyroidectomy and surgical removal of local and distant metastases where possible.
Undifferentiated or anaplastic thyroid carcinoma is a highly aggressive tumor comprised of large, spindle-shaped cells. The tumor is radiation-resistant, and extensive local as well as distant metastases generally preclude a surgical cure. Survival is limited to a few months in most cases. Of the non-epithelial thyroid tumors, lymphoma is important to identify because this tumor type may be confused with lymphocytic thyroiditis (Hashimoto’s thyroiditis). A correct diagnosis is obviously crucial to provide appropriate therapy.
Treatment of thyroid cancer
Treatment of differentiated thyroid malignancies typically includes total thyroidectomy (or partial thyroidectomy for smaller tumors), with sampling and possibly full dissection of regional lymph nodes. This may be followed by radioiodine ablation of residual thyroid tissue. Whether radioiodine is given and the amount of radioiodine given will be dictated by the estimated risk for recurrence of the thyroid cancer. After the thyroid is removed, a person will need lifelong levothyroxine replacement. In rare cases, people may require more advanced therapies for thyroid cancer such as external beam radiation or systemic chemotherapy.
Media Attributions
- Thyroid Anatomy
- Screenshot 2025-06-22 at 5.21.47 PM
- image5
- Screenshot 2025-06-22 at 5.22.45 PM
- Thyroid nodules
- Screenshot 2025-06-22 at 5.25.46 PM
