CHB Chapter Uploads
1 The Adrenal Gland
Test McPhersen
Learning Objectives
- Describe the regulation of hormone secretion from each layer of the adrenal gland
- Describe the steps in adrenal steroid synthesis, and factors that interfere with this process
- Detail the renin-angiotensin-aldosterone system (RAAS)
- Review signaling pathways of aldosterone, cortisol, adrenal androgens and epinephrine
- List the function of aldosterone, cortisol, adrenal androgens, and epinephrine and factors that stimulate and inhibit secretion these hormones
- Evaluate an adrenal nodule and list causes of adrenal hypertension
- Recognize the signs and symptoms of glucocorticoid excess, aldosterone excess, and epinephrine excess (pheochromocytoma)
- Recognize the major causes of glucocorticoid excess, aldosterone excess and pheochromocytoma
- Describe the physiologic effects of glucocorticoid excess and how this results in the clinical findings of hypercortisolism
- Select appropriate tests for diagnosis of glucocorticoid excess, aldosterone excess and pheochromocytoma
- PHARM: Identify appropriate treatment options for glucocorticoid excess, aldosterone excess and pheochromocytoma
- Identify the causes of primary and secondary adrenal insufficiency
- Recognize the symptoms of adrenal insufficiency and discuss how clinical symptoms vary between primary and secondary adrenal insufficiency.
- Select the appropriate tests for diagnosing adrenal insufficiency and distinguishing between primary and secondary
Introduction
There are two distinct endocrine organs in the adrenal gland, one surrounding the other, with completely different embryology, structure, and functions. The inner adrenal medulla, a part of the sympathetic nervous system, secretes the catecholamines epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine.
The outer adrenal glands (the cortex) secrete three hormones that help regulate the body’s response to stress. Cortisol manages the supply of fuel, from fat and muscle, while modulating the immune and inflammatory responses. Aldosterone regulates salt and water metabolism. Androgens contribute to sex steroid signaling.
Both underproduction and overproduction of adrenal hormones contribute to pathological conditions that can be fatal if left untreated. This chapter will focus on adrenal cortical glucocorticoid and androgen production, as well as the hormones of the adrenal medulla and related clinical problems.
Anatomy and function of the adrenal gland
Anatomy of the adrenal gland
Adrenal glands are located in the retroperitoneal space, immediately above the kidneys (‘ad-renal’). Both adrenals are fed by many small arteries that come from multiple larger arteries. Venules draining the adrenal cortex flow between the medullary cells (inner layer). This venous drainage allows for very high levels of cortisol produced in the cortex to reach the cells in the medulla and help maintain a key enzyme necessary for synthesis of catecholamines in the adrenal cortex. The adrenal gland is also supplied by efferent sympathetic and parasympathetic neurons. These neurons secrete norepinephrine, acetylcholine or vasoactive intestinal peptide, which primarily regulate the function of medullary cells.
Type your textbox content here.
Key Takeaways
Type your key takeaways here.
- First
- Second
Adrenal cortex morphology
The adrenal cortex is roughly divided into three layers (Figure 1). The outermost zona glomerulosa produces aldosterone. The middle zona fasciculata produces glucocorticoids. The innermost zona reticularis produces adrenal androgens. The adrenal medulla will be discussed below.
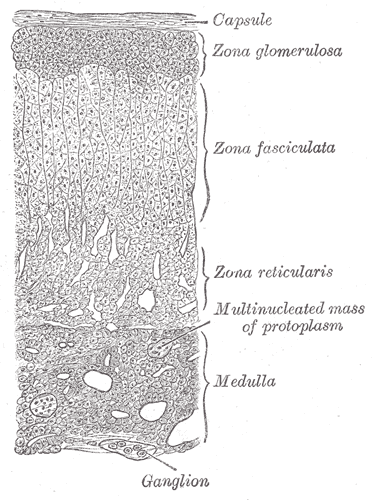
Trophic hormones, those that stimulate the release of the adrenal hormones, are needed to maintain the normal size and enzymatic function for different cortical zones. Angiotensin II, part of the renin/angiotensin cascade is the key trophic factor for the zona glomerulosa and stimulates aldosterone release. Pituitary ACTH is the key trophic factor for the zona fasciculata and zona reticularis, stimulating both cortisol and adrenal androgen production. The medulla is stimulated by sympathetic neurons.
A useful pneumonic for the anatomy and function of the different layers of the adrenal cortex is “GFR: salt, sugar, sex” (Table 1).
|
Cortical Zone |
Hormones |
Trophic factor |
Letter |
Function |
|
Glomerulosa |
Mineralocorticoids |
Angiotensin II |
G |
Salt |
|
Fasciculata |
Glucocorticoids |
ACTH |
F |
Sugar |
|
Reticularis |
Androgens |
ACTH |
R |
Sex |
Table 1. Trophic factor and function of the different layers of the adrenal gland
- Steroid synthesis, transport, and metabolism
Cholesterol is the substrate from which all steroid hormones are produced via a sequential, regulated, enzymatic cascade described below. There are multiple enzymes involved in steroid hormone synthesis; most are oxidative enzymes in the CYP (cytochrome P-450) family. This is illustrated in figure 2. The specific steps are not meant to be memorized, but you should understand the general process of synthesizing the different adrenal hormones.
Steroid synthesis starts when free cholesterol is transported from the cytosol to the inner mitochondrial membrane to be cleaved by CYP11A. This enzyme is the rate-limiting step in adrenal steroid production. CYP21 is needed for production of aldosterone and cortisol. Mutations in this enzyme are one of the most common genetically-inherited disorders in the world – causing 90% of cases of congenital adrenal hyperplasia (CAH), which is covered in detail later in the Foundations phase. Defects in this enzyme prevent production of aldosterone and cortisol and shunt all precursors into the androgen pathway. CYP11B is necessary for the final steps in mineralocorticoid and glucocorticoid production. CYP11B1 is expressed in the zona fasciculata and reticularis and regulated by ACTH. CYP11B2 is regulated by Angiotensin II and is expressed at lower levels and only in the zona glomerulosa. Mutations in CYP11B affects both pathways and are a rare cause of CAH (5-8% of cases). The production of glucocorticoids and adrenal androgens requires CYP17. Mutations in this gene lead to very high mineralocorticoid levels as all precursors are shunted through this pathway.
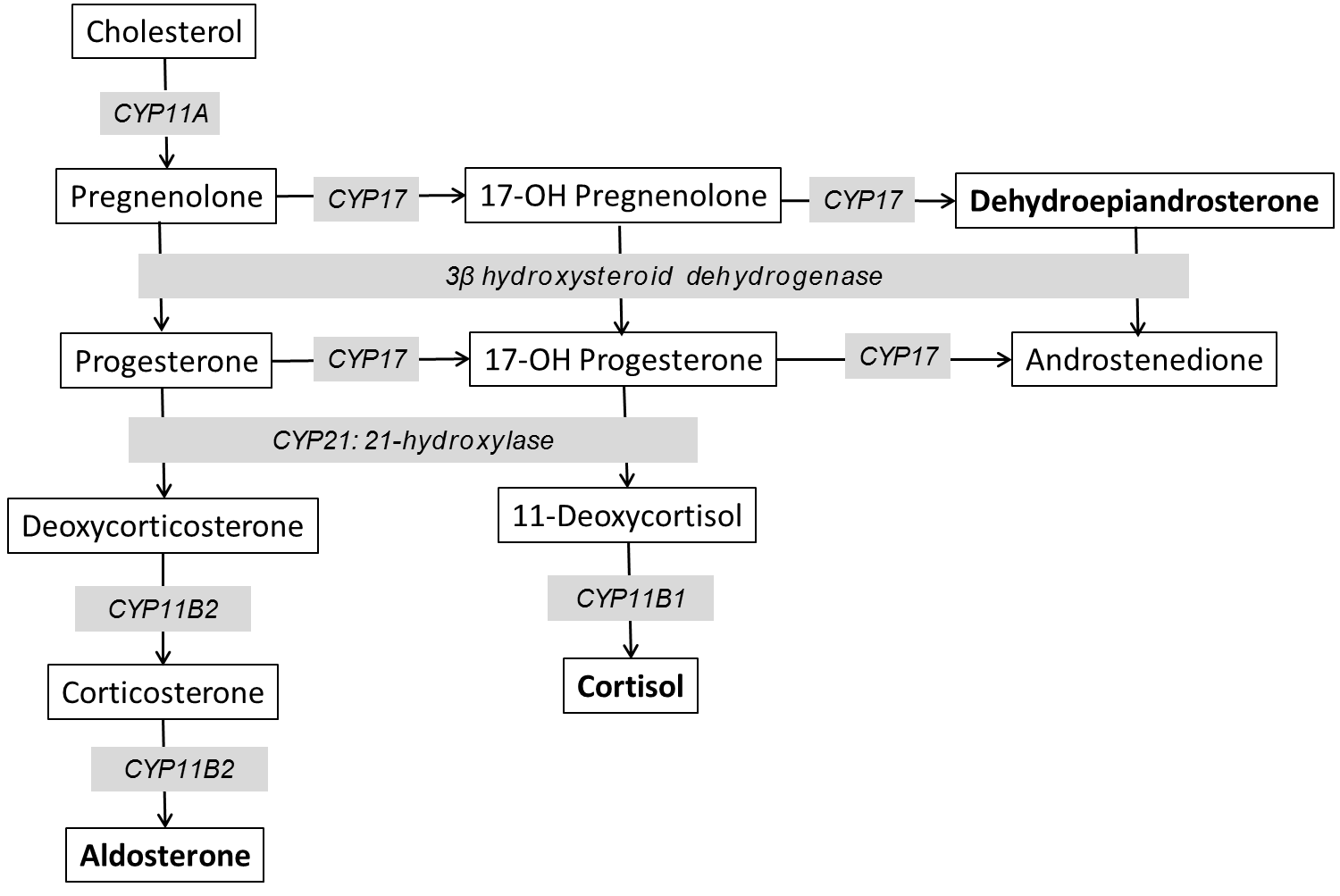
Figure 2. Diagram of adrenal steroid synthesis.
- The adrenal cortex
- Pituitary regulation
- Both glucocorticoids and adrenal androgens are regulated by a classic hypothalamic-pituitary hormonal regulatory loop (Figure 3). Mineralocorticoids are controlled by the renin-angiotensin-aldosterone pathway. In the hypothalamic-pituitary-adrenal (HPA) feedback loop, corticotrophin releasing hormone (CRH) regulates an anterior pituitary trophic/stimulating hormone (ACTH) which stimulates release of a peripheral effector hormone (cortisol) from the adrenal fasciculata which feeds back to suppress both CRH and ACTH.
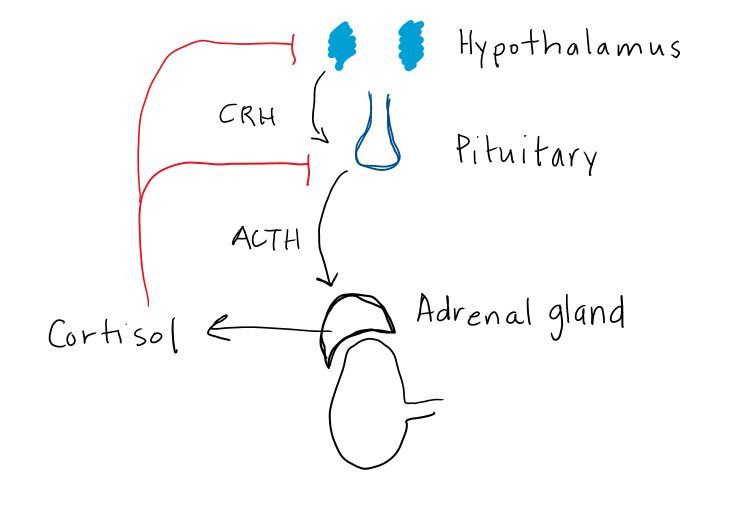
Figure 3. Hypothalamic-pituitary-adrenal feedback loop controlling cortisol secretion.
CRH is a large (41 amino acid) hypothalamic peptide that is produced in the hypothalamus. CRH stimulates ACTH release, which in turn controls cortisol and adrenal androgen secretion. Release of CRH is pulsatile with largest and most frequent pulses in the morning, which explains the normal diurnal rhythm of ACTH and cortisol release. Depression, anxiety and stress can disrupt the normal pulsatile action, leading to elevated ACTH and cortisol levels.
In response to CRH binding at the pituitary, corticotroph cells secrete a large pro-hormone, POMC which is cleaved to hormonally-active ACTH and α-melanocyte stimulating hormone (α-MSH). More on the relevance of α-MSH later in this chapter. ACTH acts at the adrenal cortex to stimulate both cortisol and androgen production. ACTH is also a trophic hormone, meaning it stimulates growth of the adrenal gland. Chronic ACTH deficiency results in atrophy of the adrenal cortex, and decreased levels of enzymes needed for cortisol production. Similarly, high levels of ACTH can lead to adrenal hypertrophy and increased enzyme activity, resulting in increased capacity of the adrenal glands to produce cortisol and enlargement of the adrenal glands.
Cortisol inhibits both CRH and ACTH release, which in turn decreases cortisol secretion (Fig 3). Synthetic glucocorticoids (aka. exogenous steroids) also inhibit CRH and ACTH. This effect is often profound due to higher potency and longer half-life of synthetic glucocorticoids compared to cortisol. The hypothalamus and pituitary can take weeks to months to fully recover normal CRH and ACTH secretion after prolonged treatment with glucocorticoid medications. As well, the adrenal glands will atrophy in the setting of chronic glucocorticoid treatment, due to absence of ACTH stimulation.
- Mineralocorticoids
Aldosterone is produced in the cells of the zona glomerulosa and secretion is primarily under the control of elevated renin and potassium levels. The renin-angiotensin-aldosterone system (RAAS) (Fig 4, described in more detail in the renal course) is complex and starts with renin secretion from the juxtaglomerular cells of the kidney leading to formation of angiotensin I which is converted to angiotensin II by the enzyme angiotensin-converting enzyme (ACE). Renin release is stimulated by hypoperfusion, hypotension, volume depletion, and increase sympathetic activity. Angiotensin II causes vasoconstriction and also leads to aldosterone release from the zona glomerulosa.
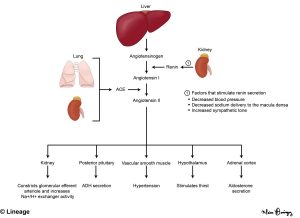
Cholesterol is the substrate for the synthesis of all steroid hormones and progesterone is the substrate for mineralocorticoid synthesis. Cholesterol conversion to pregnenolone (and then progesterone) is stimulated by renin, angiotensin II, and elevated potassium. As seen in Figure 2, progesterone is hydroxylated by enzyme CYP21A2 to yield deoxycorticosterone (DOC). The following three steps (DOC corticosterone 18-hydroxycorticosterone aldosterone) are all catalyzed by the enzyme CYP11B2 (aldosterone synthase).
Aldosterone binds to the mineralocorticoid receptor leads to sodium retention and potassium excretion by increasing the expression of sodium channels in the distal tubule of the kidney. Aldosterone also stimulates renal acid excretion. In turn, extracellular volume and blood pressure increase and blood may become more alkalotic. You will hear more on acid-base physiology later in the Foundations phase (renal and pulmonary blocks).
- Glucocorticoids
About 90% of cortisol in the circulation is protein bound: 75% to cortisol-binding globulin (CBG) and 15% to albumin. The remaining 10% circulates as free, biologically active cortisol. Changes in protein levels can affect levels of free cortisol. High levels of estrogen stimulate CBG, so pregnancy can result in high total cortisol levels but normal free cortisol levels. Oral contraceptives or exogenous estrogen can also affect levels of total cortisol. Both CBG and albumin are negative acute phase reactants – meaning the liver synthesis of these proteins decreases rapidly in the setting of systemic inflammation or severe illness. Thus, total cortisol levels are often low in critical illness, with normal or high free cortisol levels. This can make the interpretation of cortisol levels difficult in critically ill patients.
Glucocorticoids such as cortisol exert their “classical” effects by binding to specific intracellular receptors in the cytoplasm. The receptor/ligand complex translocates to the nucleus where they bind to glucocorticoid response elements (GRE) and affect gene transcription. Cortisol has equal affinity for both the glucocorticoid and the mineralocorticoid receptor (MR). However, most tissues express the enzyme 11βHSD2 which converts cortisol into inactive cortisone to prevent signaling at the MR.
Cortisol normally acts via the glucocorticoid receptor to increase fuel in times of increased stress. Almost all tissues respond to glucocorticoids. In muscle, cortisol causes net muscle breakdown, by blocking or decreasing normal anabolic signals (insulin, IGF-1). This ensures an increased supply of amino acids to the liver for gluconeogenesis during times of stress.Glucocorticoids also increase blood glucose levels by making skeletal muscle more resistant to insulin-mediated glucose uptake. Acutely, a rise in glucocorticoids promotes lipolysis in adipose with a release of free fatty acids and glycerol to be converted from fat into glucose to maintain normal blood sugar levels.
The effects of excess cortisol on the immune system are used to treat many inflammatory conditions and provide immunosuppression. Glucocorticoid excess increases circulating neutrophils and decreases circulating monocytes, eosinophils, and lymphocytes. It also decreases the migration of these inflammatory cells to sites of injury and inflammation. Furthermore, the production of lymphocytes, and mature T and B cell mediator and effector functions, are decreased.
- Adrenal androgens
DHEA is the most abundant hormone secreted from the adrenal gland. Secretion is regulated by ACTH and does not increase or decrease in response to gonadotropins or circulating testosterone or estrogen levels. Adrenal androgens—DHEA and androstenedione – are relatively weak with only 10-15% of the potency of testosterone but can be converted to testosterone and then aromatized to estradiol and estrone in the periphery. Effects of adrenal androgens are dependent on the age and sex of the individual. Tumors of the adrenal gland that secrete DHEA can cause signs of androgenization (hirsutism, masculinization or virilization) in females. It is rare to have isolated adrenal androgen excess. These tumors typically co-secrete cortisol and are typically malignant.
- The Adrenal Medulla
- Anatomy and embryology of the medulla
Chromaffin cells, the catecholamine-producing cells of the medulla, comprise about 10% of the weight of each adrenal gland (about 0.5 gm). Chromaffin cells are embryologically derived from the neural crest. Extra-adrenal chromaffin cells may be found in the paravertebral and preaortic regions. The adrenal gland has several arteries penetrating the outer cortex and a central vein exiting the adrenal hilum. A portal system arises from the adrenal cortex (where cortisol, adrenal androgens, and aldosterone are made) and supplies blood to the medulla where catecholamines are synthesized. Adrenal portal blood glucocorticoid levels are 100 times higher than levels in the general circulation. Blood flow to the medulla is neurally regulated and increases under physiologically stressful conditions to release catecholamines into the general circulation. The adrenal medulla is innervated by preganglionic sympathetic fibers that stimulate secretion of catecholamines.
peptides go here

Feedback/Errata