3 Acetycholine receptors
Learning Objective 3: Compare nicotinic and muscarinic acetylcholine receptor activation and identify acetylcholine receptor types and their effects on various target organs.
Distinguishing nicotinic and muscarinic ACh receptorsThere are two major categories of AChRs: nicotinic (nAChR) and muscarinic (mAChR). Except that they both live in the plasma membrane and bind ACh, nicotinic and muscarinic receptors are quite different in structure, function, and pharmacology. Both have many isoforms (genes).
Nicotinic nAChRs are fast, ligand-gated cation channels opened directly by binding of ACh. Opening these channels can depolarize a cell to 0 mV. [Technically, being permeable to Na+ and K+, their reversal potential is around 0 mV.] Outside the central nervous system, nACHRs are located both at the skeletal neuromuscular junction (NMJ) and at synapses onto postganglionic neurons in all ANS ganglia and onto adrenal chromaffin cells. The nAChR isoforms (genes) expressed in nerve versus muscle are different, so it is possible to use drugs to block the neuronal and muscle receptors selectively. However, many nAChR pharmacological agents will affect both, including ACh, which is the natural agonist for both. The ACh binding sites of nAChRs are blocked by the paralytic drug curare and related compounds. The nicotinic name comes from the early discovery that nicotine and smoke from tobacco can activate these receptors as well. There are no nAChRs on cardiac muscle, smooth muscle, or on non-nerve/muscle tissue cells such as liver, kidney, epithelia, etc.
Muscarinic mAChRs are G-protein coupled receptors (GPCRs), not ion channels. They are located on the target tissues of parasympathetic innervation and at the sympathetic sweat gland—indeed on most cells of the body. There are five types, but the two primary receptor types to consider here are M2 and M3. All are inhibited by the drug atropine (an antagonist competing with ACh at the binding site) from deadly nightshade. Atropine does not affect nicotinic receptors.
M2 couples to GαiSlows heart rate through both:
1) activation of a K+ channel via Gβγ subunits, leading to hyperpolarization of pacemaker, and
2) turning off the formation of the stimulatory cAMP second messenger through inhibiting adenylyl cyclase via Gαi.
M3 couples to GαqThe following three actions all result from the ability to elevate intracellular Ca2+ through Gq and the PLC/IP3 pathway.
• Contracts smooth muscle
• Stimulates glands to secrete (including eccrine sweat glands)
• Elevates nitric oxide (NO) production in endothelial cells leading to smooth muscle relaxation (vasodilation)
Synthesis and cycling of ANS neurotransmittersWe now review synthesis and degradation of the neurotransmitters E/NE and ACh (see also ANS Pharmacology sessions):
NE and E are catecholamines, members of the biogenic amine family. They are released from postganglionic varicosities, taken up again by a Na+/Cl−-coupled NE uptake transporter, and degraded by cytoplasmic monoamine oxidase (MOA).
You do NOT need to memorize their chemical structures.
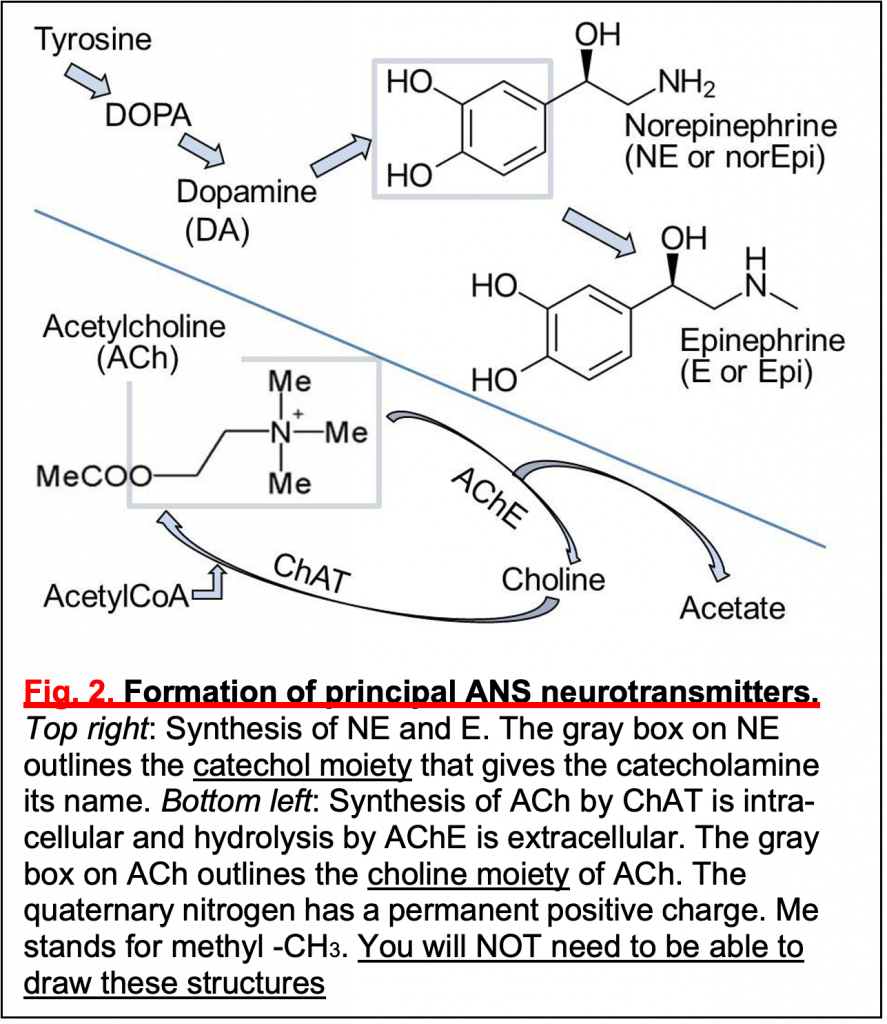
• Synthesis (Fig.2): The precursor tyrosine is taken up into varicosities by a Na+/tyrosine cotransporter. Cytosolic enzymes convert tyrosine to DOPA and then to dopamine.
• Dopamine is transported into secretory vesicles.
• Inside vesicles, dopamine is converted to NE.
• In the adrenal gland, NE leaks out again to the cytosol where NE is methylated to form E (Fig.2), and E is retransported into the giant secretory vesicles called “chromaffin granules” in the adrenal gland.
NE is normally released from vesicles by exocytosis, but NE also leaks from nerve into serum. This leak is increased if cytoplasmic monamine oxidases (MAOs) are inhibited by MAO inhibitors (MAOIs).
ACh is an ester formed from choline and acetate (Fig.2). Choline and Acetyl-CoA are combined by choline acetyl transferase (ChAT) in the presynaptic terminal. ACh is then transported into secretory vesicles and released at varicosities and synaptic terminals. ACh is inactivated in the extracellular space by hydrolysis back to choline and acetate by the highly active extracellular enzyme acetylcholin- esterase (AChE) present in all cholinergic tissues. The enzyme AChE is the target of nerve gases, such as CX, and organophosphate insecticides. Death is by accumulation of excess circulating ACh and, for humans, by exaggerated parasympathetic action. Choline is recycled by uptake into the nerve by a Na+-coupled cotransporter–conservation. There, choline acetyl transferase resynthesizes ACh. Of all the steps mentioned here, the AChE enzyme will be the most important one for you to remember now.
You do NOT need to remember the structures.
